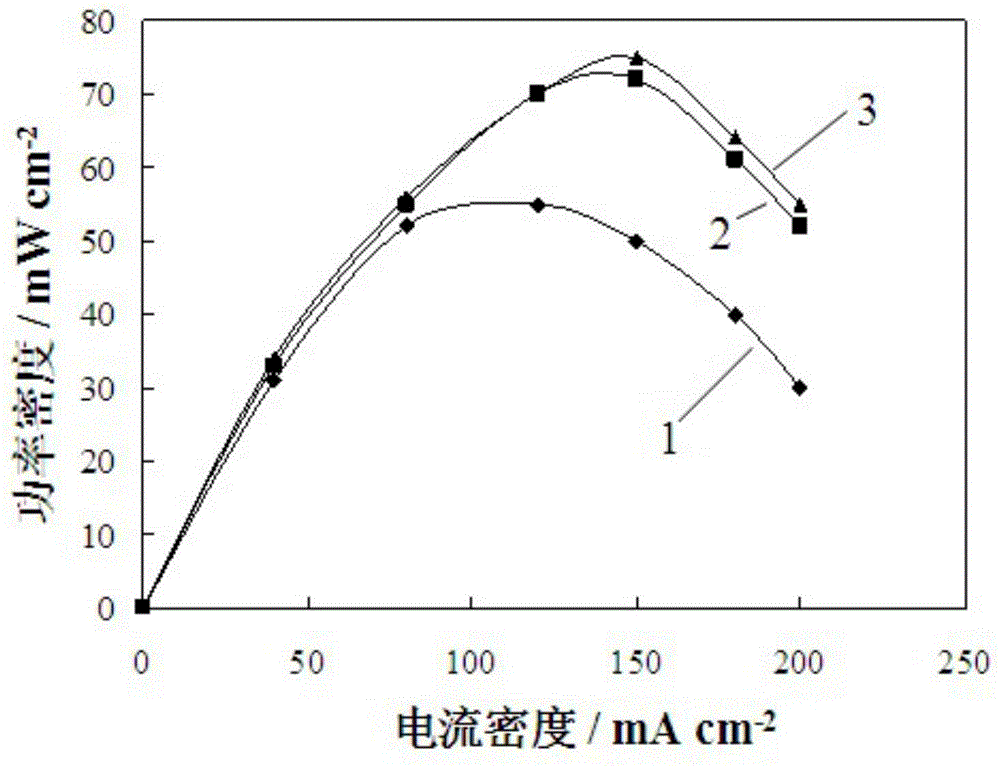
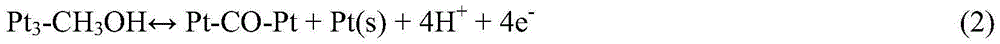Method for modifying direct methanol fuel cell anode catalyst
A methanol fuel cell and catalyst technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as low performance, and achieve the effects of improving catalytic activity, high catalytic activity, and reducing internal resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of tungsten oxide modified sulfur-containing macroporous carbon
[0037] Take glucose, thiourea and ammonium tungstate and add them into deionized water, and mix well to form a solution; wherein, the molar ratio of glucose to thiourea is 1:1, the molar ratio of thiourea to water is 1:100, thiourea and tungsten The mol ratio of ammonium acid is 1: 0.5;
[0038] Polymerize the solution at 90°C for 30 minutes to form a thiourea-glucose resin, add hydrophilic nano-calcium carbonate, and form a uniform suspension after stirring; wherein the mass ratio of thiourea-glucose resin to calcium carbonate is 1:1; After the turbid liquid was spray-dried, in flowing N 2 Put it in a tube furnace under protection, and heat it at 200°C, 650°C and 1000°C for 2 hours respectively to obtain sulfur-containing macroporous carbon modified with tungsten oxide;
Embodiment 2
[0039] Example 2: Preparation of tungsten carbide modified sulfur-containing macroporous carbon
[0040] Add sucrose, thiourea and ammonium tungstate to deionized water, and mix well to form a solution; wherein, the molar ratio of sucrose and thiourea is 1:1, the molar ratio of thiourea and water is 1:100, and the molar ratio of thiourea and tungsten The mol ratio of ammonium acid is 1: 0.3;
[0041] Polymerize the solution at 90°C for 30 minutes to form thiourea-sucrose resin, add hydrophilic nano-calcium carbonate, and form a uniform suspension after stirring; wherein the mass ratio of thiourea-sucrose resin to calcium carbonate is 1:1; After the turbid liquid was spray-dried, in flowing N 2 Put it in a tube furnace under protection, and heat it at 200°C, 650°C and 2000°C for 2 hours respectively to obtain tungsten carbide-modified sulfur-containing macroporous carbon.
Embodiment 3
[0042] Example 3: Preparation of tungsten oxide-modified sulfur-containing macroporous carbon-supported platinum catalyst
[0043] Add water-soluble starch, thiourea and ammonium tungstate to deionized water, and mix well to form a solution; wherein, the molar ratio of water-soluble starch to thiourea is 1:1, the molar ratio of thiourea to water is 1:100, and the molar ratio of sulfur to water is 1:100. The molar ratio of urea and ammonium tungstate is 1:0.2;
[0044] Polymerize the solution at 90°C for 30 minutes to form thiourea-starch resin, add hydrophilic nano-calcium carbonate, and form a uniform suspension after stirring; wherein the mass ratio of thiourea-starch resin and calcium carbonate is 1:1; After the turbid liquid was spray-dried, in flowing N 2 Put it in a tube furnace under protection, and heat it at 200°C, 650°C and 1000°C for 2 hours respectively to obtain sulfur-containing macroporous carbon modified with tungsten oxide;
[0045] Add chloroplatinic acid (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com