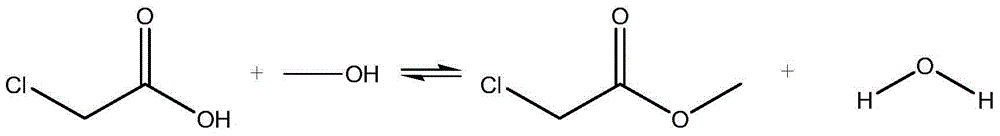
Reactive distillation method for preparing methyl chloroacetate
A technology of methyl chloroacetate and reactive distillation, which is applied in the field of reactive distillation for preparing methyl chloroacetate, can solve the problems of high process energy consumption, secondary pollution, complicated distillation process, etc., and achieves simple production process, No secondary pollution, high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
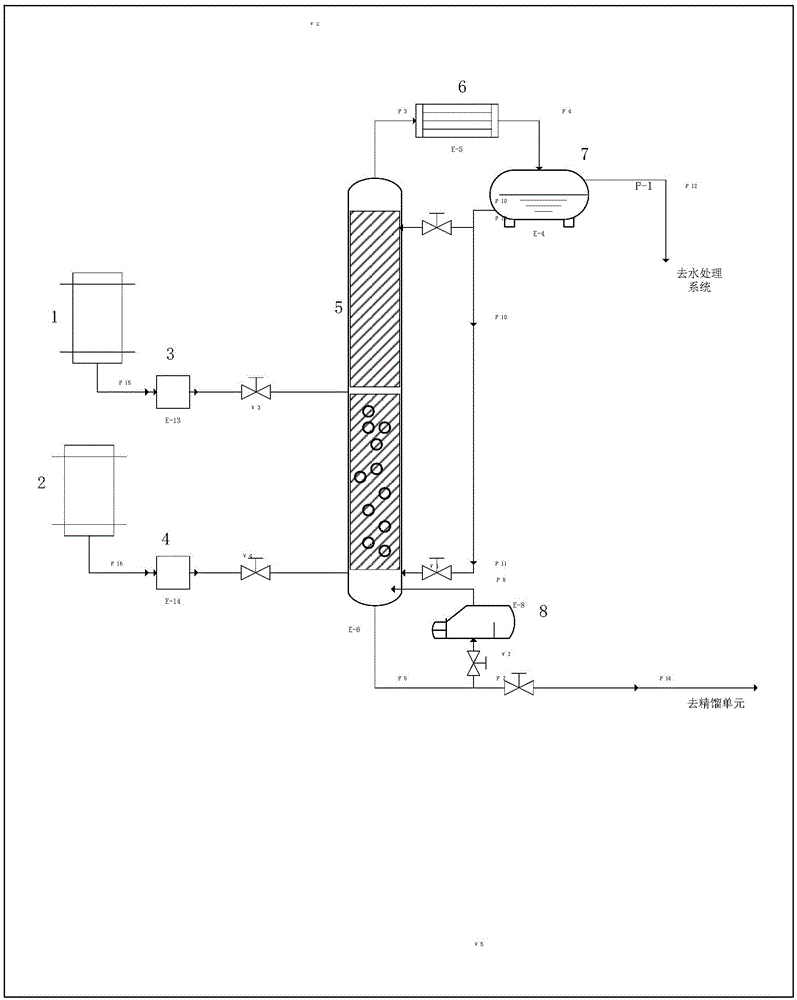
[0031] New method flow chart of the present invention sees figure 1 , chloroacetic acid and methanol are stirred and mixed in the raw material tank 1 at 25°C according to the molar ratio of 0.3:1, and injected into the top of the reaction section of the reactive distillation tower through the raw material pump 3, and the middle part of the reactive distillation tower is pyridine trifluoromethanesulfonate The acid polymerization ionic liquid catalyst and filler are mixed, and the upper layer and the lower layer are equipped with seasoning. The temperature of the tower bottom is controlled at 120°C, and the temperature of the tower body is controlled at 110°C. The gas-phase mixed product is condensed by the condenser at the top of the reactor, and then enters the ester-water separator, and the content of each component in the upper and lower layers is detected by gas chromatography. The lower layer is divided into two paths, part of which flows back to the top of the tower with...
Embodiment example 2
[0033] New method flow chart of the present invention sees figure 1 , chloroacetic acid and methanol are stirred and mixed in the raw material tank 1 at 25°C according to the molar ratio of 0.4:1, and injected into the top of the reaction section of the reactive distillation tower through the raw material pump 3, and the middle part of the reactive distillation tower is pyridine trifluoromethanesulfonate The acid polymerization ionic liquid catalyst and filler are mixed, and the upper layer and the lower layer are equipped with seasoning. The temperature of the tower bottom is controlled at 125°C, and the temperature of the tower body is controlled at 115°C. The gas-phase mixed product is condensed by the condenser at the top of the reactor, and then enters the ester-water separator, and the content of each component in the upper and lower layers is detected by gas chromatography. The lower layer is divided into two paths, part of which flows back to the top of the tower with...
Embodiment example 3
[0035] New method flow chart of the present invention sees figure 1 , chloroacetic acid and methanol are stirred and mixed in the raw material tank 1 at 25°C according to the molar ratio of 0.5:1, and injected into the top of the reaction section of the reactive distillation tower through the raw material pump 3, and the middle part of the reactive distillation tower is pyridine trifluoromethanesulfonate The acid polymerization ionic liquid catalyst and filler are mixed, and the upper layer and the lower layer are equipped with seasoning. The temperature of the tower bottom is controlled at 130°C, and the temperature of the tower body is controlled at 120°C. The gas-phase mixed product is condensed by the condenser at the top of the reactor, and then enters the ester-water separator, and the content of each component in the upper and lower layers is detected by gas chromatography. The lower layer is divided into two paths, part of which flows back to the top of the tower with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com