Pyroimidazole derivative, preparation method thereof and electroluminescent device
An electroluminescent device and technology of imidazole derivatives, applied in the field of organic electroluminescence, can solve the problems of no light emission, reduction of luminous efficiency, quenching, etc., and achieve strong fluorescence properties, simple synthesis method, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: cis-1,1'-2phenyl-2,2'-2tetraphenylethenyl-4,5,9,10-pyrenimidazole (M1)
[0029]
Embodiment 2
[0030] Example 2: trans-1,1'-2phenyl-2,2'-2tetraphenylethenyl-4,5,9,10-pyrenimidazole (M2)
[0031]
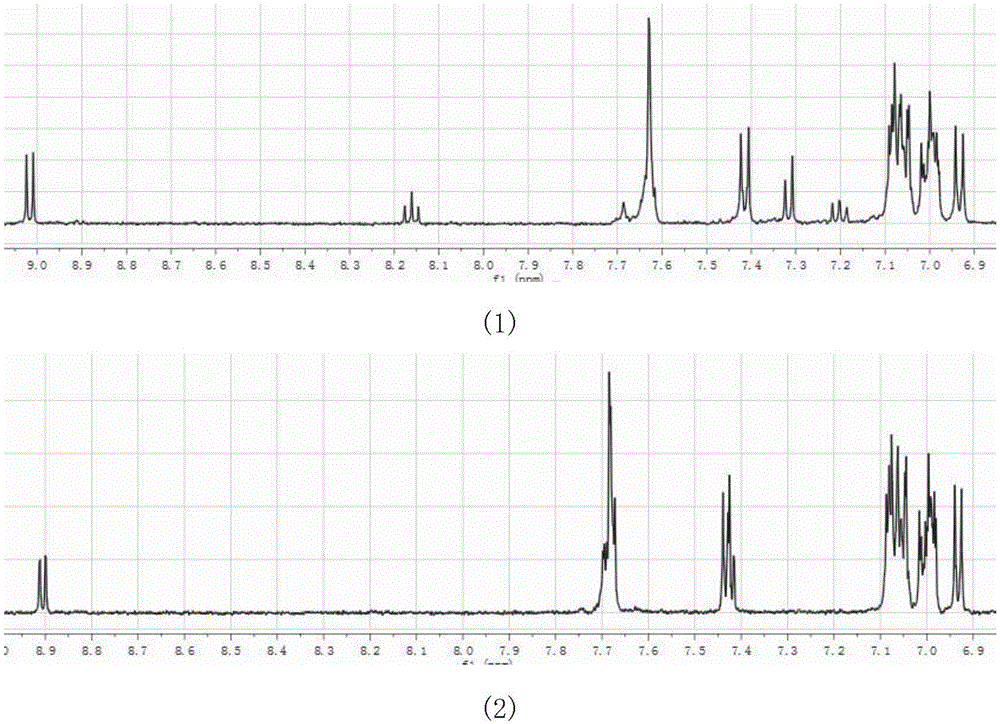
[0032] Heat an appropriate amount of pyrenequinone (0.5g), tristyrylbenzaldehyde (1.6g), aniline (2ml) and ammonium acetate (1.5g) in 15ml of glacial acetic acid to reflux at 120°C for two hours, then stop heating and cool After reaching room temperature, filter to obtain dark green solid, adopt SiO 2 Column separation, dichloromethane:petroleum ether volume ratio of 1:1 and dichloromethane were successively purified through the column to obtain 0.45g of the green target product (Example 1), with a yield of 22%. Such as figure 1 As shown in (1), 1 H NMR (500MHz, d8-THF, ppm): 9.02(d,2H), 8.16(t,1H), 7.62(m,10H), 7.41(d,4H), 7.32(d,2H), 7.20(t ,1H), 7.12-7.03(m,18H), 7.03-6.97(m,12H), 6.93(d,4H). Mass spectrometry data (C 82 h 54 N 4 ) theoretical value: 1095.33; measured value: 1096.8. Elemental analysis (C 82 h 54 N 4 ) theoretical value: C: 89.92; H: 4.97; N: 5...
Embodiment 3
[0034] Embodiment 3: the preparation of electroluminescent device
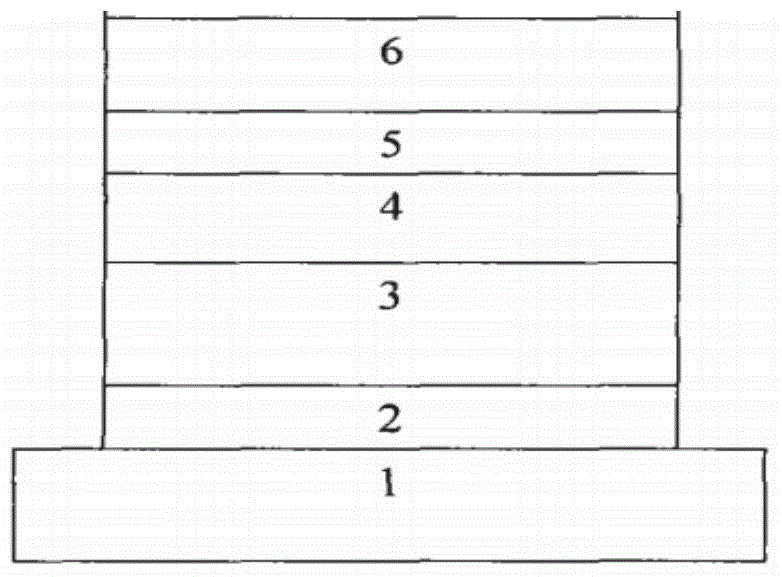
[0035] Such as figure 2 The device structure shown includes: a substrate layer 1 , a hole injection layer 2 , a hole transport layer 3 , a light emitting layer 4 , an electron transport layer 5 , and a cathode layer 6 . The substrate layer 1 is glass and the conductive layer attached to it is indium tin oxide (positive electrode, ITO, 40nm), the hole injection layer 2 is polyethylenedioxythiophene (PEDOT, 40nm), and the hole transport layer 3 is 4,4 '-bis(N-phenyl-N-naphthyl)-biphenyl (NPB, 40nm), the light emitting layer 4 is a pyrenimidazole compound (30nm), and the electron transport layer 5 is 1,3,5-tri(1 -Phenyl-1H-benzimidazol-2-yl)benzene (TPBi, 50nm), the cathode layer 6 is a metal layer (LiF / Al, 0.75nm / 100nm).
[0036] The electroluminescent device is prepared according to methods known in the art, such as the fabrication method disclosed in reference (Adv. Mater. 2003, 15, 277.). The specific met...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com