Support for antibody purification, manufacturing method for same, and application for same
A technology for immobilizing carriers and antibodies, applied in the field of affinity chromatography columns, which can solve the problems of loss of antibody activity, change of high-level structure of antibodies, and development needs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0207] gene synthesis
[0208] The genes described in the examples were synthesized by a contract manufacturer of synthetic genes. dsDNA was synthesized based on the nucleotide sequence (SEQ ID NO: 8) shown below, and inserted into the BamHI-EcoRI site of the pUC18 vector; the sequence of the resulting clone was confirmed by single-strand analysis, and nucleotide sequence Cross-checking of information; mutation correction of sites identified as mismatches by methods such as site-directed mutagenesis; and delivery of resulting obtained plasmid DNA (approximately 1 μg). The sequence of the expected portion of the delivered plasmid was reconfirmed by sequencing.
[0209] Nucleotide sequence of SEQ ID NO:8
[0210] GGATCCTTGACAATATCTTAACTATCTGTTATAATATATTGACCAGGTTAACTAACTAAGCAGCAAAAGGAGGAACGACTATGGCGGATAACAACTTTAACCGCGAACAGCAGAACGCGTTTTATGAAATTCTGAACATGCCGAACCTGAACGAAGAACAGCGCAACGGCTTTATTCAGAGCCTGCGCGATGATCCGAGCCAGAGCGCGAATCTGCTGAGCGAAGCGCGTCGTCTGAATGAAAGCCAGGCGCCGGGCTGTGCGGATGA...
Embodiment 2
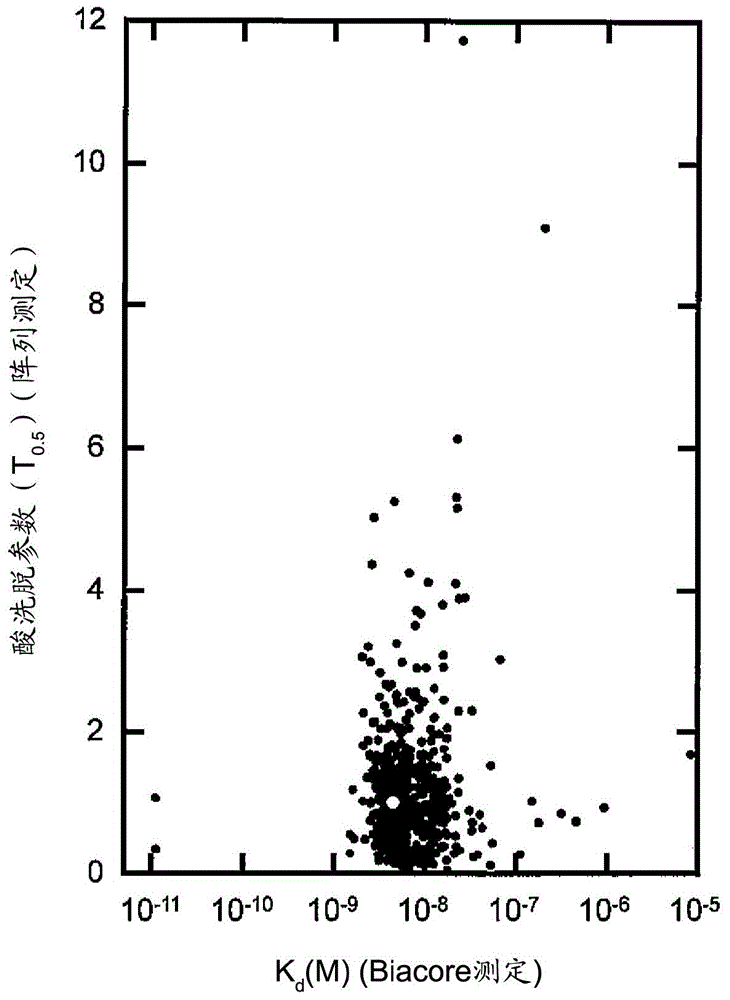
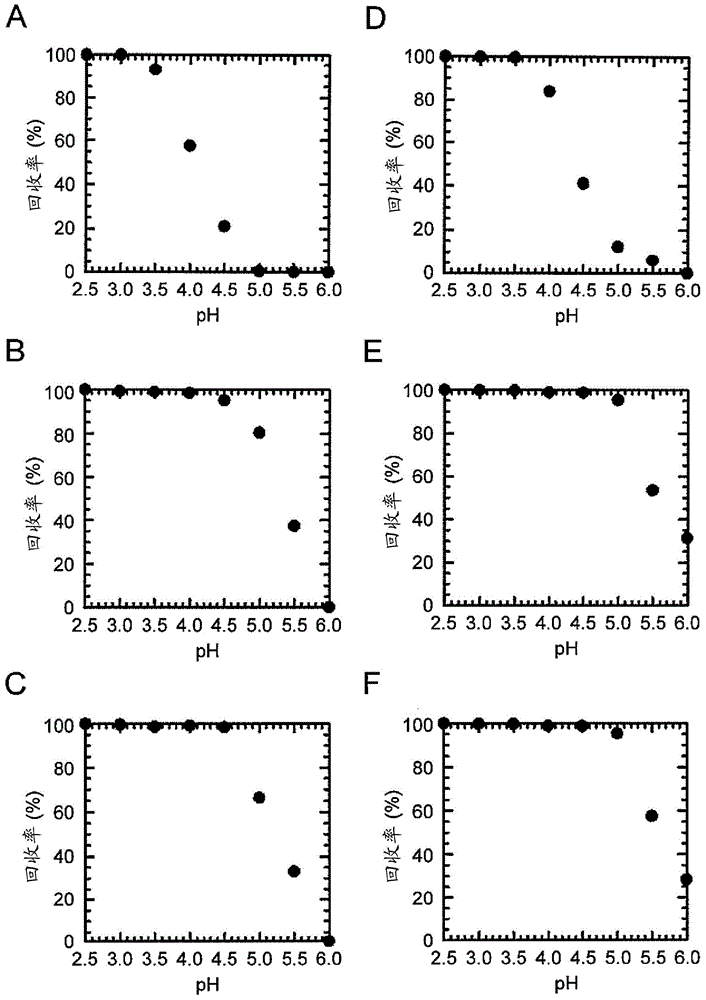
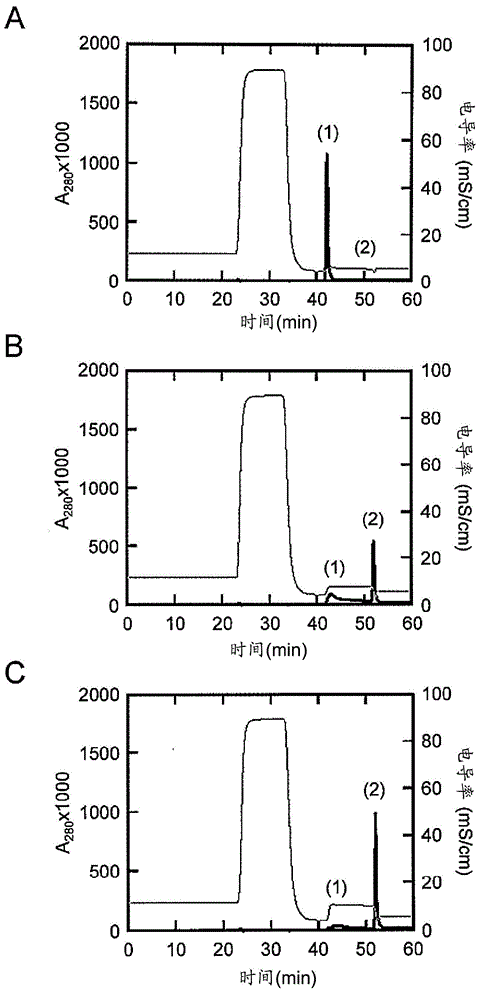
[0234] Based on the results of Example 1, other single amino acid substitution mutations were further added to the E15H mutation. As added mutations, the positions M19 (methionine at position 19 in the amino acid sequence shown in SEQ ID NO: 1) and G29 (methionine at position 29 in the amino acid sequence shown in SEQ ID NO: 1) were checked. Substitution mutation at the glycine at the position. This is because M19 is the only methionine residue present in AD-1 and is sensitive to air oxidation, and amino acid substitution at this site is expected to increase antioxidant properties as a chemical property. Position G29 is the position shown as one of the positions whose mutation is expected to improve the elution characteristics at pH 5.0 as a result of the array analysis. For site M19, M19V (replacing methionine with V (valine) at position 19) was selected as the mutation, where T as a parameter for ease of elution at pH 5 0.5 was improved, and for position G29, G29A (substit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com