Magnetic nanohydrogel and preparation method thereof
A nano-hydrogel, magnetic nano-technology is applied in the field of nano-hydrogel to achieve the effects of improving magnetic signal, simple process and improving clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Dissolve 0.9710g N,N-diethylacrylamide DEA, 0.0292g acrylic acid AA, 0.0603g N,N'-methylenebisacrylamide and 0.0502g sodium lauryl sulfate in 95g deionized water , and bubbling N at room temperature 2 Deoxygenation, magnetic stirring for 120 minutes;
[0032] (2) The reaction temperature rises to 70°C, under N 2 Insulate the above solution under protection for 30 minutes;
[0033] (3) then prepare the ammonium persulfate solution that mass concentration is 0.4%, get 4.9987g and add in the above-mentioned solution, keep N 2 atmosphere, continue to react for 4 hours;
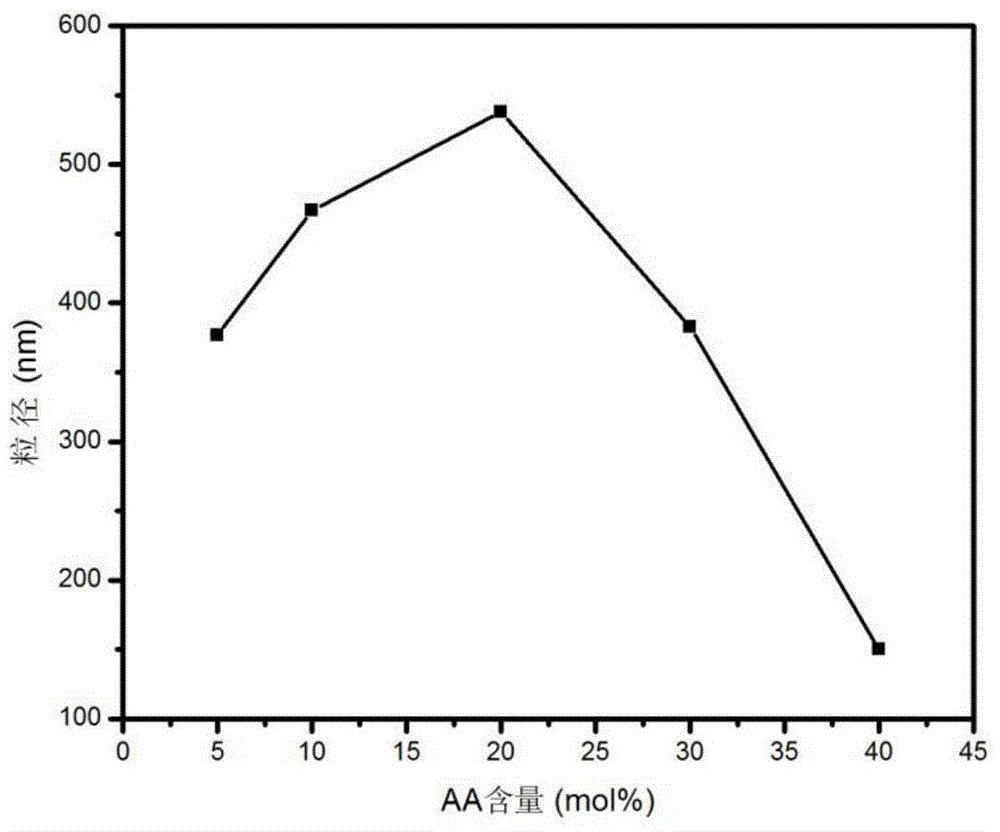
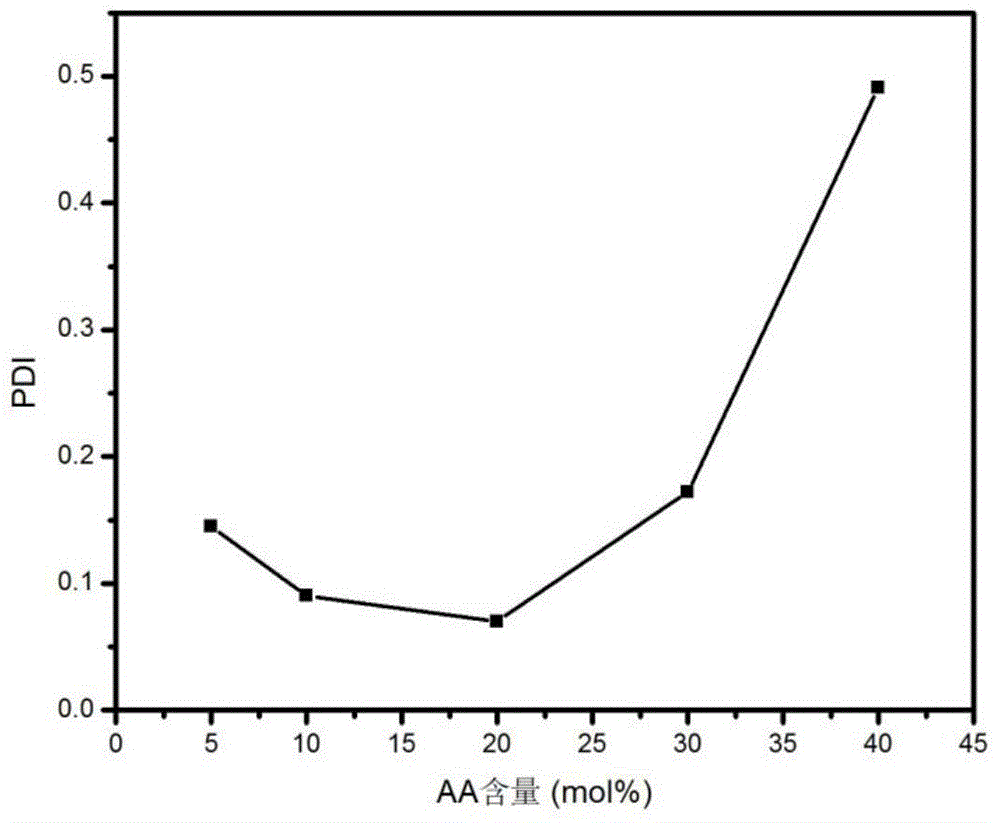
[0034](4) Then soak the obtained reactant in deionized water for dialysis for 4 days, change the water twice a day, remove the residual reaction raw materials and the electrolyte in the reaction system, the molecular weight cut-off of the dialysis bag used is 8000~14000, and the AA content is 5mol%. Copolymerized hydrogels were sampled for DLS testing.
Embodiment 2
[0036] (1) Dissolve 0.9409g DEA, 0.0587g AA, 0.0605g N,N'-methylene bisacrylamide and 0.0497g sodium lauryl sulfate in 95g deionized water, and blow N at room temperature 2 Deoxygenation, magnetic stirring for 120 minutes;
[0037] (2) The reaction temperature rises to 70°C, under N 2 Insulate the above solution under protection for 30 minutes;
[0038] (3) then prepare the ammonium persulfate solution that mass concentration is 0.4%, get 5.0004g and add in the above-mentioned solution, keep N 2 atmosphere, continue to react for 4 hours;
[0039] (4) Then soak the obtained reactant in deionized water for dialysis for 4 days, change the water twice a day, remove the residual reaction raw materials and the electrolyte in the reaction system, the molecular weight cut-off of the dialysis bag used is 8000~14000, and the AA content is 10mol%. Copolymerized hydrogels were sampled for DLS testing.
Embodiment 3
[0041] (1) Dissolve 0.1243g DEA, 0.8758g AA, 0.0601g N,N'-methylene bisacrylamide and 0.0502g sodium lauryl sulfate in 95g deionized water, and blow N at room temperature 2 Deoxygenation, magnetic stirring for 120 minutes;
[0042] (2) The reaction temperature rises to 70°C, under N 2 Insulate the above solution under protection for 30 minutes;
[0043] (3) then preparation mass concentration is the ammonium persulfate solution of 0.4%, gets 5.0006g and adds in the above-mentioned solution, keeps N 2 atmosphere, continue to react for 4 hours;
[0044] (4) Soak the obtained reactant in deionized water and dialyze for 4 days, change the water twice a day, remove the residual reaction raw materials and the electrolyte in the reaction system, the molecular weight cut-off of the dialysis bag used is 8000~14000, and the AA content is 20mol% Copolymerized hydrogels were sampled for DLS testing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com