Preparation method of graphite oxide phase carbon nitride modified electrode and application of electrode in detection of heavy metal ions
A technology of graphite phase carbon nitride and electrode modification, applied in the direction of electrochemical variables of materials, etc., can solve the problems of film formation, easy stacking of lamellae, difficult compounding, etc., and achieves improved compatibility, large specific surface area, and improved dispersion. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
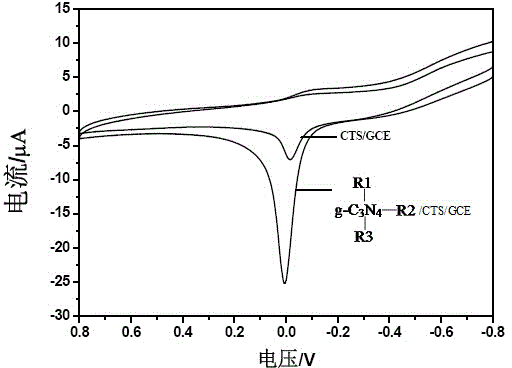
Image
Examples
preparation example Construction
[0032] The preparation method of graphite oxide phase carbon nitride modified electrode comprises the following steps:
[0033] First, the preparation of graphite oxide phase carbon nitride:
[0034] (1) Add concentrated sulfuric acid in an ice-water bath, add graphite phase carbon nitride under a magnetic stirrer, the solution turns yellow, then slowly add potassium permanganate, time after the addition, and react for 2-3 hours under stirring Hour, the solution becomes dark brown, and the controlled reaction temperature is no more than 5°C;
[0035] (2) The dark brown solution obtained in step (1) continued to stir and react in a water bath at 35-45 °C, and a large number of bubbles were generated. After reacting for 1 hour, slowly add deionized water to dilute;
[0036](3) Add 1-2 mol / L hydrogen peroxide to the diluent obtained in step (2) for reduction, and strictly control the reduction temperature at 35-45 °C until the solution is milky white;
[0037] (4) Continue to f...
Embodiment 1
[0049] 1. Synthesis steps of graphite oxide phase carbon nitride:
[0050] (1) A certain amount of melamine was weighed and placed in a tube furnace, calcined at 550 °C for 2 h at a heating rate of 5 °C / min, and ground to obtain powdery carbon nitride with light yellow graphite structure (g - C 3 N 4 ).
[0051] (2) Add 46 ml of concentrated sulfuric acid in an ice-water bath, and add 2 g of graphite-phase carbon nitride under a magnetic stirrer.
[0052] (3), slowly add 6 g KMnO in step 2 4 , Timing after addition, reacted for 3 hours under stirring, the solution was dark brown.
[0053] (4) Transfer the beaker in step 3 to a water bath at a temperature of 35 °C, continue magnetic stirring, slowly add 200 ml of deionized water for dilution, control the temperature not to exceed 40 °C, and react for 1 h.
[0054] (5), slowly add 5% hydrogen peroxide to the beaker in step 4 for reduction, the solution is milky white. Strain while hot. Fully washed with 5% HCl and deionize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com