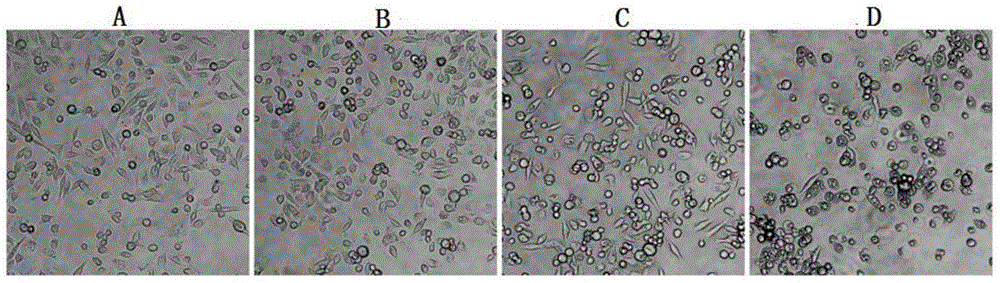
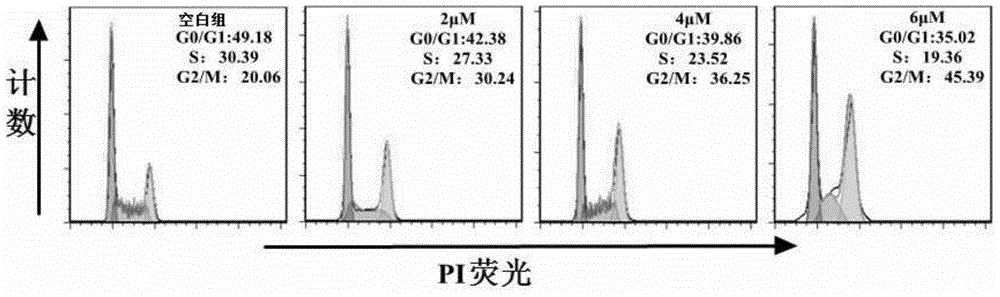
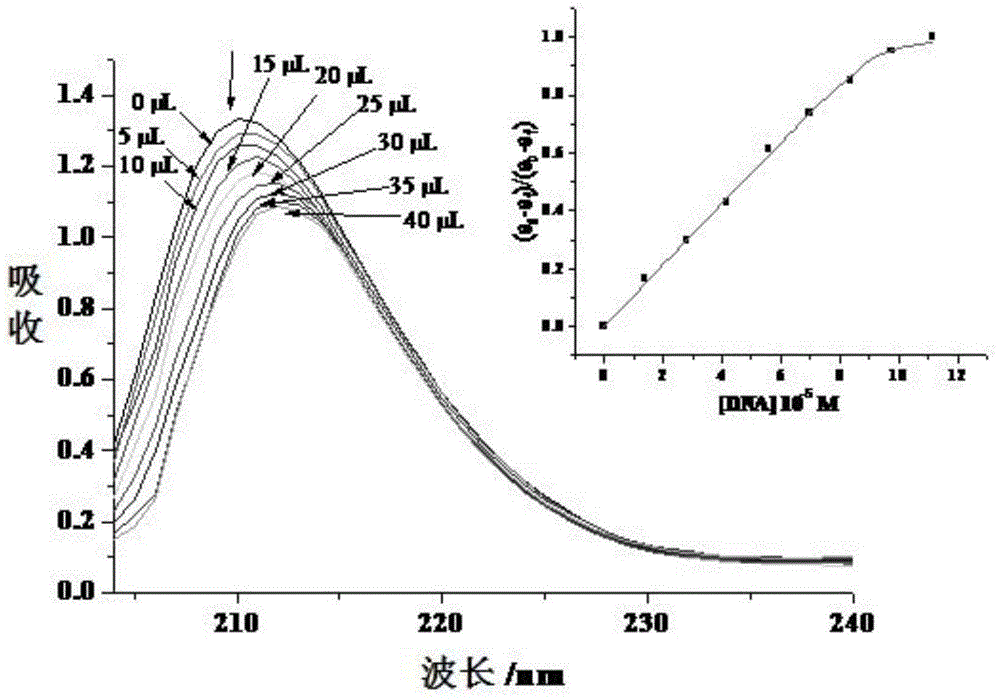
Ruthenium complex as well as preparation method and use thereof
A technology of ruthenium complexes and ligands, which can be used in ruthenium organic compounds, pharmaceutical formulations, platinum group organic compounds, etc., can solve the problems of cisplatin with large toxic and side effects, achieve good absorption effect, induce tumor cell apoptosis, and be beneficial to The effect of industrial synthesis and preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 Preparation of ruthenium complexes of the present invention
[0028] The ruthenium complex in this embodiment has the following structure:
[0029]
[0030] The above-mentioned ruthenium complex is synthesized from imidazole as a raw material through a three-step reaction, and the synthetic route is as follows:
[0031]
[0032] In this embodiment, R is -H.
[0033] Its preparation method is specifically:
[0034] (1) Preparation of Compound III
[0035] 1mol imidazole, 2mol K 2 CO 3 1mol benzyl chloride was dissolved in acetone, heated to reflux for 8h, and then the obtained mixed solution was filtered to remove insoluble matter, and the obtained filtrate was spin-dried by a rotary evaporator, and then 10:1 CH 2 Cl 2 with CH 3 OH was used as the eluent for chromatographic column separation to obtain a light yellow solid, which was compound III;
[0036] (2) Preparation of Compound II
[0037] Dissolve 1 mol of compound III obtained in step (...
Embodiment 2
[0041] Embodiment 2 Preparation of ruthenium complexes of the present invention
[0042] The ruthenium complex in this embodiment has the following structure:
[0043]
[0044] The synthetic route of above-mentioned ruthenium complex is identical with embodiment 1. The preparation method of the ruthenium complex described in this example is the same as that in Example 1, the only difference is that in the preparation steps of compound III, benzyl chloride is replaced by 4-methoxybenzyl chloride, and the preparation steps of compound I are as follows: :
[0045] Take 2mol of the NHC ligand shown in formula II and 1mol of Ag 2 O was stirred in 10ml of dichloromethane in the dark for 4 hours, and then added 1mol of dichlorobis(4-methylisopropylphenyl)ruthenium (II), and continued to stir in the dark at 18°C until the reaction was obtained The ruthenium complex.
[0046]
[0047] Among them, R is -OCH 3 .
Embodiment 3
[0048] Embodiment 3 Preparation of ruthenium complexes of the present invention
[0049] The ruthenium complex in this embodiment has the following structure:
[0050]
[0051]The synthetic route of above-mentioned ruthenium complex is identical with embodiment 1. The preparation method of the ruthenium complex described in this example is the same as that in Example 1, the only difference is that in the preparation steps of compound III, benzyl chloride is replaced by 4-methylbenzyl chloride, and the preparation steps of compound I are as follows:
[0052] Take 2mol of the NHC ligand shown in formula II and 1mol of Ag 2 O was stirred in 10ml of dichloromethane in the dark for 4h, then added 1mol of dichlorobis(4-methylisopropylphenyl)ruthenium (II), and continued to stir in the dark at 28°C until the reaction was obtained The ruthenium complex; after the reaction, the above solution was filtered to remove the by-product AgCl, and then 40ml of ether was added to the filtr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com