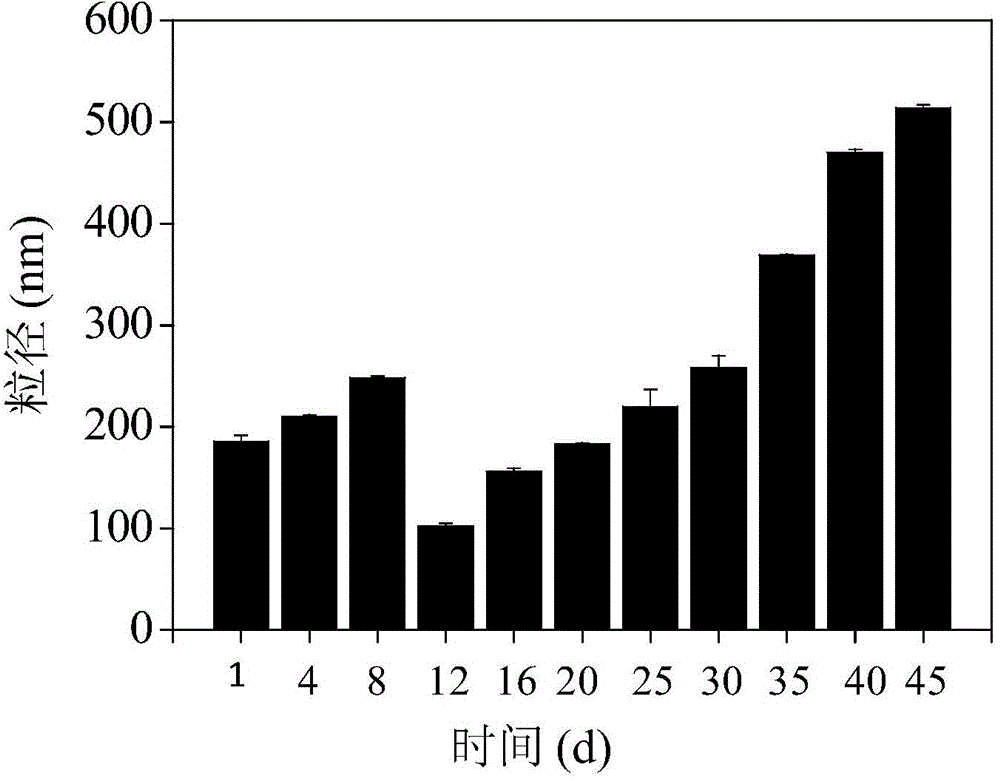
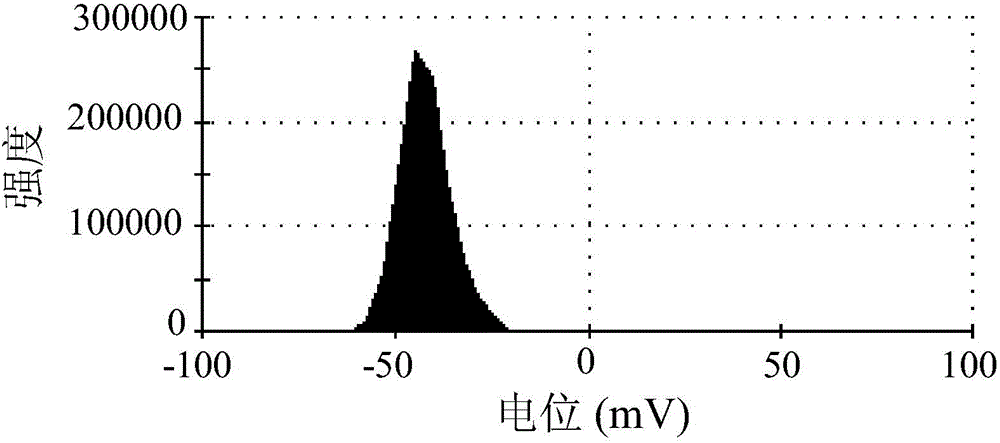
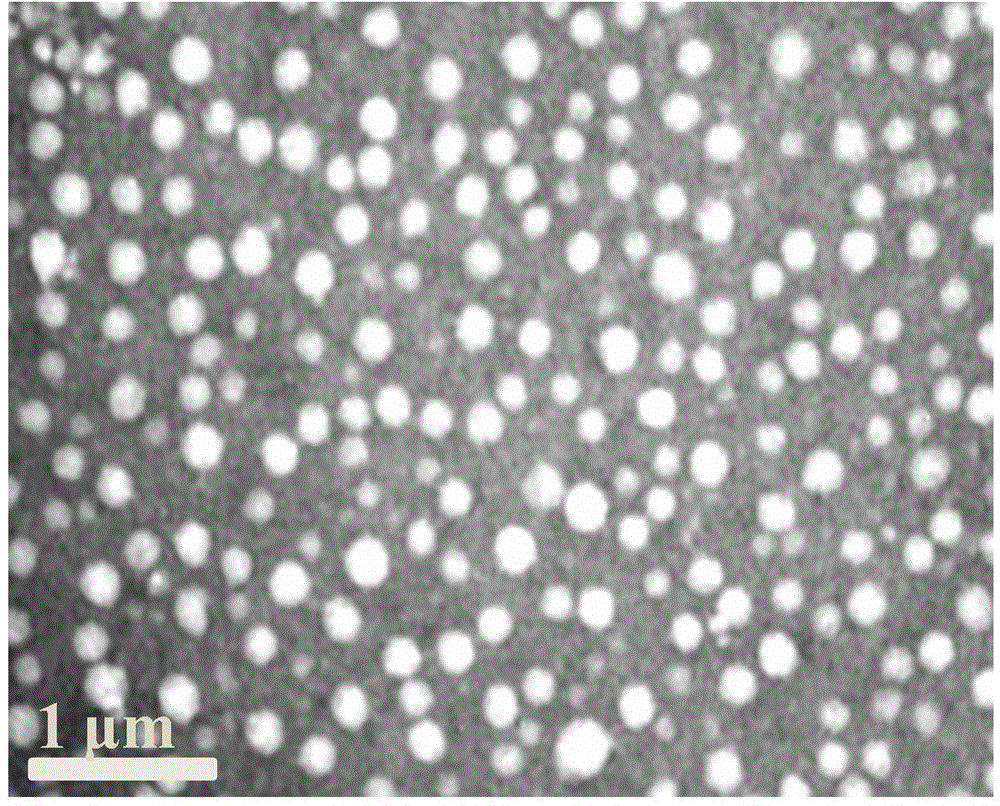
Antitumor drug carrier and application method thereof
An anti-tumor drug and carrier technology, applied in the field of biomedicine, can solve the problems of severe systemic toxicity and low drug concentration, and achieve the effects of convenient storage, good biocompatibility and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The above-mentioned phycobiliprotein is made into phycobiliprotein nanoparticles of the antitumor drug carrier of the present invention, and the preparation method comprises the following steps:
[0067] S1, prepare phycobiliprotein aqueous solution;
[0068] S2. Use a pH regulator to adjust the pH of the phycobiliprotein aqueous solution away from the isoelectric point of the phycobiliprotein;
[0069] S3. Desolventizing phycobiliproteins from the aqueous phycobiliprotein solution to form nanoparticles;
[0070] S4. Using a cross-linking agent to cross-link the precipitated phycobiliprotein nanoparticles to obtain an aqueous solution of tightly cross-linked phycobiliprotein nanoparticles.
[0071] Wherein step S1 is specifically, using the dry protein powder of the above-mentioned phycobiliprotein, dissolving it in water, and the concentration of the phycobiliprotein aqueous solution is 2-200 mg / mL.
[0072] Wherein step S2 is specifically, adjusting the pH of the abov...
Embodiment 1
[0089] Embodiment 1 Preparation of Phycobiliprotein Nanoparticles
[0090] Under normal temperature and pressure (15-35°C, 1 standard atmospheric pressure), prepare a water-soluble phycoerythrin solution with a mass concentration of 2 mg / mL, and adjust the pH of the phycoerythrin solution to 7.0 with 0.1N aqueous sodium hydroxide solution. Slowly add 8 mL of absolute ethanol dropwise under continuous stirring at a rate of 0.5 mL / min to desolventize the phycoerythrin to form a nano-protein sol. After the dropwise addition, 23.5 μL of 8% (volume fraction) glutaraldehyde was added, mixed evenly under a magnetic stirrer, and the reaction was continued for 1 hour. After the reaction, the phycoerythrin nanoparticles were centrifuged for 2 rounds (the centrifugation speed was 5000rpm, and each round lasted 10 minutes), and then resuspended with 8 mL of double distilled water to obtain a colloidal suspension of phycoerythrin nanoparticles, which was denoted as PCNPs .
[0091] Under...
Embodiment 2
[0097] Example 2 Phycobiliprotein Nanoparticle Loaded Drugs
[0098] At normal temperature and pressure (15-35°C, 1 standard atmospheric pressure), prepare a water-soluble phycocyanin (PC) solution with a mass concentration of 20 mg / mL, and adjust the pH of the phycocyanin solution to 8.4. Slowly add 8 mL of anhydrous acetone dropwise under continuous stirring at a rate of 0.8 mL / min to desolvate the phycocyanin to form a nano-protein sol. After the dropwise addition, 23.5 μL of 8% (volume fraction) vanillin was added, mixed evenly under a magnetic stirrer, and the reaction was continued for 24 hours. After the reaction, the phycocyanin nanoparticles were centrifuged for 5 rounds (the centrifugation speed was 12000rpm, and each round lasted 10 minutes), and then resuspended with 8 mL of double distilled water to obtain a colloidal suspension of phycocyanin nanoparticles, which was recorded as PCNPs.
[0099] Dissolve 16mg of folic acid (folate, FA) in 0.8mL of 0.1N sodium hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com