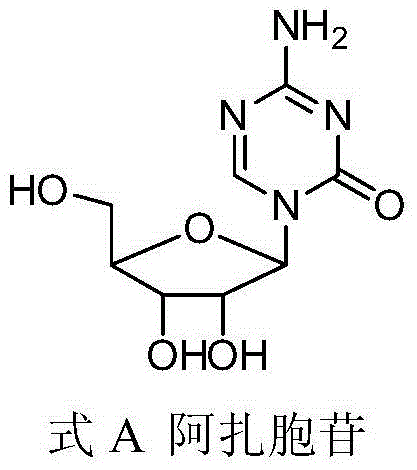
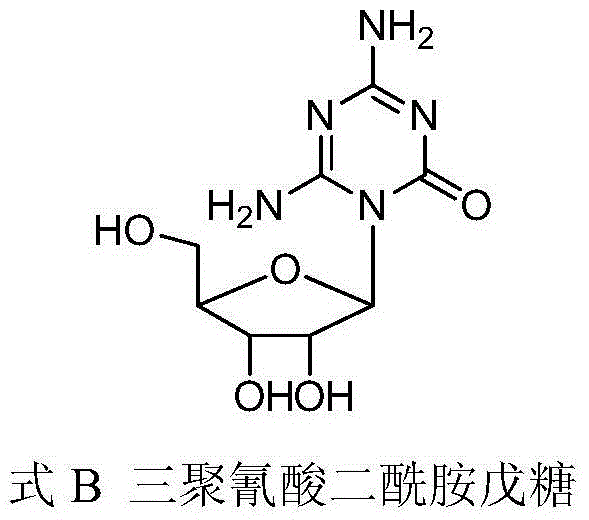
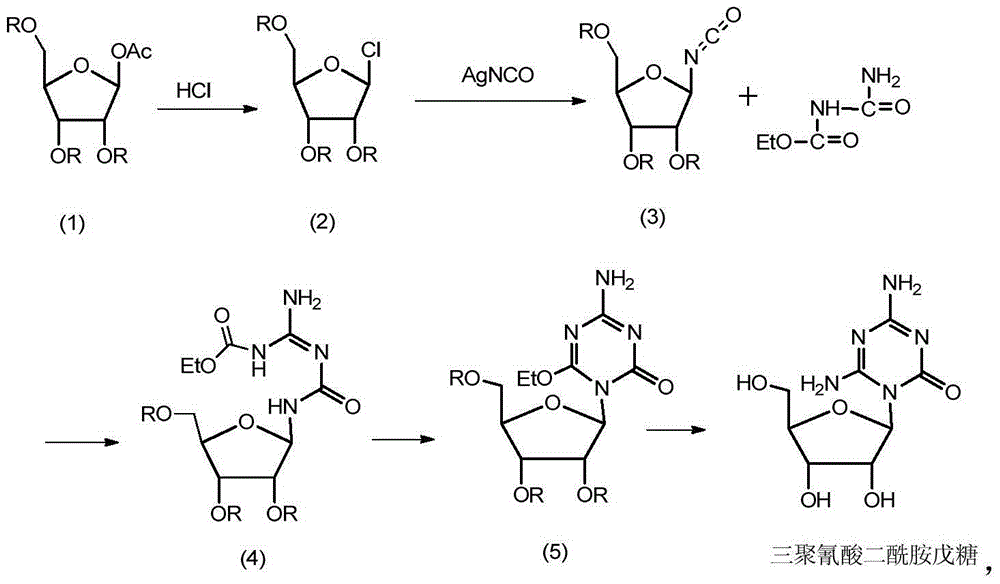
Preparation method of azacitidine impurity
A technology of ammeline pentose and compound, which is applied in the field of medicinal chemistry and can solve the problems of long synthesis steps, low yield and complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 N 2 ,N 4 - Preparation of bis(trimethylsilyl)-6-trimethylsiloxy-1,3,5-triazine-2,4-diamine
[0037]
[0038] Weigh 2.54g of 6-amino-5-azacytosine into a 100mL three-neck flask, add 50.2ml of hexamethyldisilazane (HMDS) and 50mg of ammonium sulfate, stir and heat to reflux, react for 10h, and stop after the solution is clarified After heating, the reaction solution was evaporated to dryness under reduced pressure to obtain the title compound.
[0039] ESI-MS m / z:[M+H] + =344.2.
Embodiment 2
[0040] Example 2 (2R,3R,4R,5R)-2-(acetoxymethyl)-5-(4,6-diamino-2-oxo-1,3,5-triazine-1(2H )-yl)tetrahydrofuran-3,4-diyl diacetate
[0041]
[0042] Weigh 6.86g (0.02mol) of the compound prepared in Example 1 into a 250ml four-neck flask, add 80ml of dichloromethane and 5.72g (0.018mol) of tetraacetyl ribose, stir, cool down to about 0°C, and add AlCl 3 A total of 3.2g (0.024mol), after 5 hours of reaction at 0~20°C, pour the reaction solution into 100ml of cold saturated sodium bicarbonate solution, separate the liquids, take the organic phase, and wash it twice with cold water (50ml / time×2times) ), the organic phase was taken, dried over anhydrous sodium sulfate, filtered, and purified by column chromatography to obtain 2.31 g of the title compound, yield: 33.3%.
[0043] 1 H NMR (500MHz, DMSO-d 6)δ: 7.48 (2H), 6.87 (1H), 6.72 (1H), 5.81 (1H), 5.60 (1H), 5.46 (1H), 4.32 (1H), 4.13 (2H), 2.02-2.05 (9H).
[0044] ESI-MS m / z:[M+H] + =386.1.
Embodiment 3
[0045] Example 3 (2R,3R,4R,5R)-2-(benzoyloxymethyl)-5-(4,6-diamino-2-oxo-1,3,5-triazine-1 (2H)-yl)tetrahydrofuran-3,4-diyldibenzoate
[0046]
[0047] Weigh 6.86g (0.02mol) of the compound prepared in Example 1 into a 250ml four-neck flask, add 80ml of dichloromethane and 11.32g (0.02mol) of tetrabenzoyl ribose, stir, cool down to below 5°C, and add AlCl 3 A total of 4g (0.03mol), after 6.5h reaction at 0~15°C, pour the reaction solution into 100ml cold saturated sodium bicarbonate solution, separate the liquids, take the organic phase, wash twice with cold water (50ml / time×2times) ), the organic phase was taken, dried over anhydrous sodium sulfate, filtered, and purified by column chromatography to obtain 4.27 g of the title compound, yield: 37.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com