Application of anhydroicaritin in preparation of medicine for treating anaemia
A technology of icariin and drugs, applied in the field of medicine, can solve problems such as unsatisfactory effects, low drug compliance rate, long treatment time, etc., achieve increased red blood cell count and hemoglobin value, and low side effects and adverse reaction rates , the effect of high medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
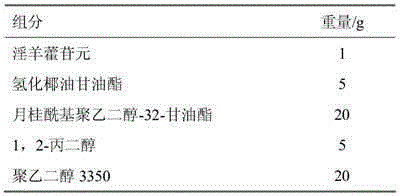
[0032] Embodiment 1 icarigenin microemulsion preparation
[0033]
[0034] Preparation process: Weigh the prescription amount of hydrogenated cocoglyceride, lauroyl polyethylene glycol-32-glyceride, 1,2-propylene glycol, polyethylene glycol 3350, mix and stir evenly, then add icariin to dissolve , Ultrasonic treatment to accelerate the dissolution, to obtain a clear concentrate, which is the icarigenin microemulsion concentrate. The microemulsion concentrate obtained above is diluted with water according to the weight ratio of 1:10-20 to a clear solution to obtain the microemulsion. The particle size was measured by a laser particle size analyzer, and the average particle size was 40nm.
Embodiment 2
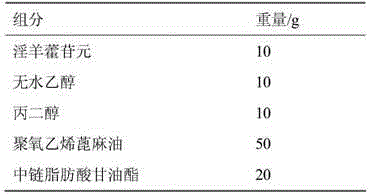
[0035] Example 2 Icarigenin enteric-coated soft capsule preparation
[0036] Contents Prescription:
[0037]
[0038] Rubber prescription:
[0039]
[0040] Enteric coating solution prescription:
[0041]
[0042] Preparation process: Weigh the prescription amount of medium-chain fatty acid glycerides, polyoxyethylene castor oil, propylene glycol, and absolute ethanol, mix and stir evenly, then add icarigenin to dissolve, or use ultrasonic treatment to speed up the dissolution, and obtain clarification and concentration The liquid is the icariin microemulsion concentrate. Dilute the microemulsion concentrate obtained above with water at a weight ratio of 1:10-20 to a clear solution to obtain the microemulsion content. Weigh the gelatin, glycerin, and purified water in the prescription amount, mix them evenly, and press them into rubber, then weigh Eudragit L30D-55, triethyl citrate, talcum powder, and purified water in the recipe amount, and mix them evenly to make...
Embodiment 3
[0043] Example 3 Icarigenin Oral Liquid
[0044]
[0045] Preparation process: Place the prescribed amount of methylparaben and ethanol in a suitable container, add the prescribed amount of icariin, heat it in a water bath to dissolve, then dissolve the prescribed amount of sodium benzoate, essence, and sucrose with a small amount of water Finally, add it to the above-mentioned drug-containing mixed solution, add water to the full amount, stir evenly, filter, pack and sterilize.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com