Screening method of anticancer medicine
A screening method and anti-cancer drug technology, applied in biochemical equipment and methods, microbe determination/testing, biological testing, etc., to achieve high sensitivity and broad prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
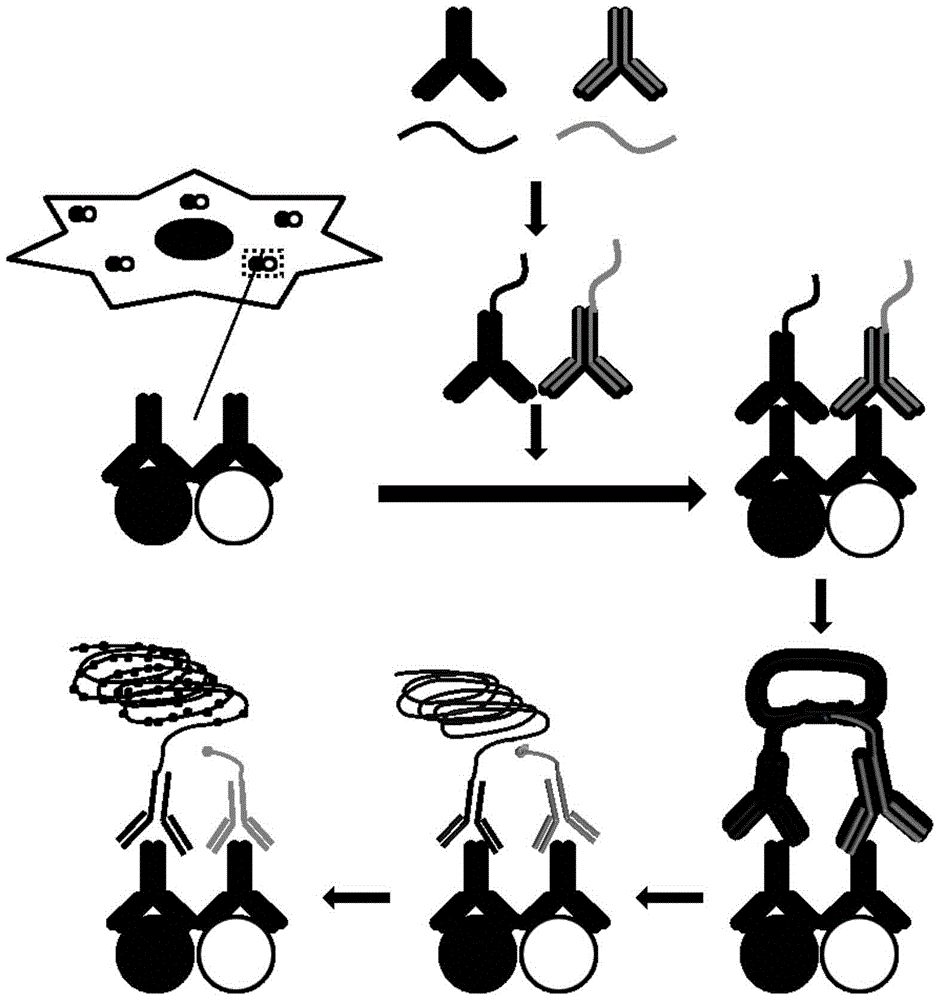
[0020] Example 1 Probes for Synthetic Oligonucleotide Chain Conjugated Antibodies
[0021] (1) Add 1 μL 4mM DBCO (Dibenzylcyclooctyne, dibenzylcyclooctyne) to 2 μg / μL 10 μL anti-mouse IgG antibody and react at room temperature for 30 minutes to add NHS groups to the N-terminal; (2) Add 1 μL 1M Tris-HCl (trishydroxymethylaminomethane-hydrochloric acid buffer) was incubated at room temperature for 5 minutes to terminate the reaction; (3) the antibody modified with NHS group and the synthesized single-chain Arm1 modified with azide group at the 5' end (SEQ ID NO:1) 1.5 μL was incubated overnight at 4°C to couple the anti-mouse IgG antibody to Arm1 to form probe 1.
[0022] Similarly, Arm2 (SEQ ID NO: 2) was added to the anti-rabbit IgG antibody to form probe 2.
Embodiment 2
[0023] Example 2 Cell culture and drug treatment
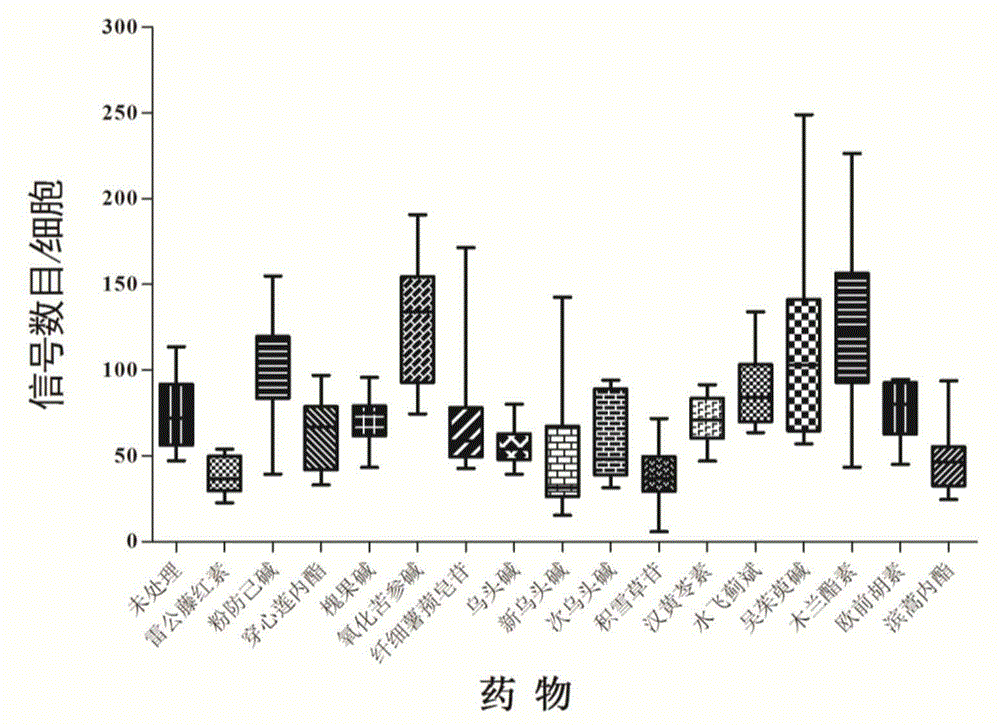
[0024] (1) Incubate the Panc1 pancreatic cancer cell line in a retort flask at 37°C, 5% CO 2 For culture, the medium is DMEM + 10% serum + 1% penicillin-streptomycin mixed solution; (2) After the cells are full, digest the cells with 0.25% trypsin and count the cells; (3) A circular glass piece with a diameter of 10 mm was placed in a 24-well cell culture plate and rinsed with DMEM; (4) Add 500 μL of 2×10 to each well of the cell culture plate 5 cells / ml, 37°C, 5% CO 2Cultivate overnight to make the cells adhere to the wall; (5) On the second day, add the drugs to be screened at a final concentration of 10 μM to each well, such as tripterine, tetrandrine, andrographolide, sophocarpine, and oxymatrine , slender diosgenine, aconitine, neoaconitine, hypoaconitine, asiaticoside, hanhuanglingsu, silymarin, evodiamine, magnolan, imperatorin, basartone, And set up a negative control. Treat the cells at 37°C for 1 hour; (6) Take...
Embodiment 3
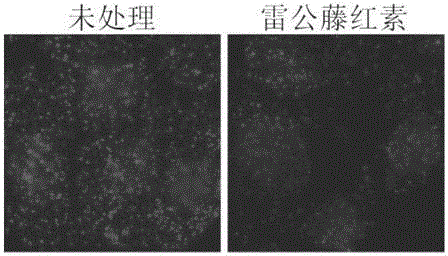
[0025] Example 3 Detection of Hsp90-Cdc37 Interaction at the Cellular Level Using In Situ Proximity Ligation
[0026] (1) Add 50 μl blocking solution (1×PBS (phosphate buffer), add 5 mM EDTA (ethylenediaminetetraacetic acid), 20% sheep serum, 2.5 μg / mL salmon sperm ( Salmon Sperm Deoxyribonucleic acid), 25mM cysteine, 0.1% Tween20) at 37°C for blocking for 2h; (2) Add rabbit antibody specific to Hsp90 and mouse antibody specific to Cdc37, overnight at 4°C; (3) Wash with TBST Three times, add 1:50 times diluted probe 1 and probe 2, and incubate at 37°C for 1h; (4) Wash three times with TBST, add long single-stranded ligated DNA (SEQ ID NO:3) and short single-stranded ligated DNA (SEQ ID NO:3) ID NO: 4), ligation reaction buffer and T4 ligase, react at 37°C for 30 minutes; (5) wash three times with TBST, add DNA polymerase and reaction buffer, react at 37°C for 90 minutes; (6) wash three times with TBST, add fluorescence Detect single-stranded DNA (SEQ ID NO:5) and nuclear dy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com