Tandospirone micropore osmotic pump preparation
A technology of tandospirenone and osmotic pump, which is applied in the directions of medical preparations containing active ingredients, drug delivery, organic active ingredients, etc., can solve the requirements of high technical level, complicated preparation process, and high performance requirements of production equipment, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Tablet prescription:
[0099]
[0100]
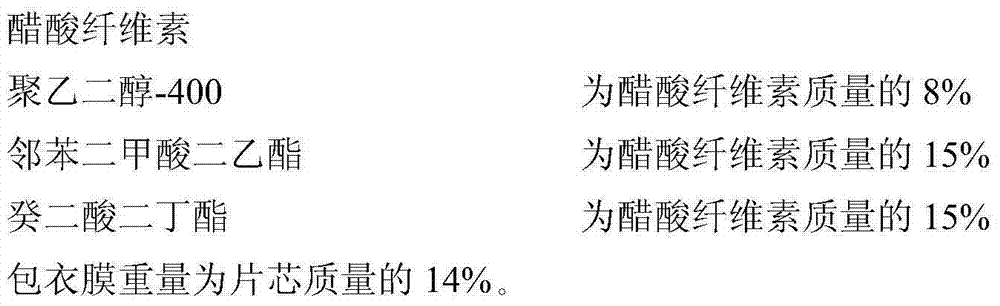
[0101] Coating Solution Prescription:
[0102]
[0103] Preparation Process:
[0104] Pass the tandospirone citrate, sodium chloride, sucrose, hydroxypropyl cellulose, sodium carboxymethyl cellulose, lactose and microcrystalline cellulose of the amount to be prepared through an 80 mesh sieve and mix thoroughly, and add an appropriate amount of 5 The ethanol solution of % povidone k15 is used to make soft materials; the soft materials are granulated with a 30-mesh sieve; after drying, 20-mesh granules are granulated, and then magnesium stearate is added and mixed; the prepared granules are compressed with a tablet machine.
[0105] Dissolve the raw materials of cellulose acetate, polyethylene glycol-400, diethyl phthalate and dibutyl sebacate, which make up the controlled-release film coat, in a mixed solvent composed of acetone and isopropanol. The core was coated with a controlled-release film, and the weight of the c...
Embodiment 2
[0110] Tablet prescription:
[0111]
[0112]
[0113] Coating Solution Prescription:
[0114]
[0115] Preparation Process:
[0116] Pass the tandospirone citrate, sucrose, malic acid, hydroxypropyl cellulose, sodium carboxymethyl cellulose, lactose and microcrystalline cellulose in the amount to be prepared through a 80-mesh sieve and mix thoroughly, adding an appropriate amount of 5% The ethanol solution of povidone k15 is used to prepare soft material; the soft material is granulated with a 30-mesh sieve; after drying, it is granulated with a 20-mesh sieve, and then magnesium stearate is added and mixed evenly; the prepared granules are compressed with a tablet machine.
[0117] Dissolve the raw materials of cellulose acetate, polyethylene glycol-400, diethyl phthalate and dibutyl sebacate, which make up the controlled-release film coat, in a mixed solvent composed of acetone and isopropanol. The core was coated with a controlled-release film, and the weight of ...
Embodiment 3
[0122] Tablet prescription:
[0123]
[0124]
[0125] Coating Solution Prescription:
[0126]
[0127] Preparation Process:
[0128] Pass the tandospirone citrate, sucrose, malic acid, povidone k30, sodium carboxymethylcellulose, lactose and dextrin in the amount to be prepared through a 80-mesh sieve and mix thoroughly, and add an appropriate amount of 5% povidone K15 ethanol solution to make soft material; soft material is granulated with 30 mesh sieve; after drying, granulate with 20 mesh, then add magnesium stearate and mix well; compress the prepared granule with tablet machine.
[0129] The raw materials of cellulose acetate, polyethylene glycol-400, diethyl phthalate and tributyl citrate, which make up the controlled-release film coat, are dissolved in a mixed solvent composed of acetone and isopropanol. Perform controlled-release film coating, increase the weight of the coating to 12% of the tablet core mass, and cure at 45°C for 12 hours.
[0130] Accordi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com