Method for producing urea peroxide
A technology for urea peroxide and a production method, applied in chemical instruments and methods, preparation of organic compounds, chemical industry and other directions, can solve the problems of low degree of automation, inability to continuously produce, and difficulty in production, and achieve automatic control, The effect of avoiding secondary energy consumption and saving raw material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
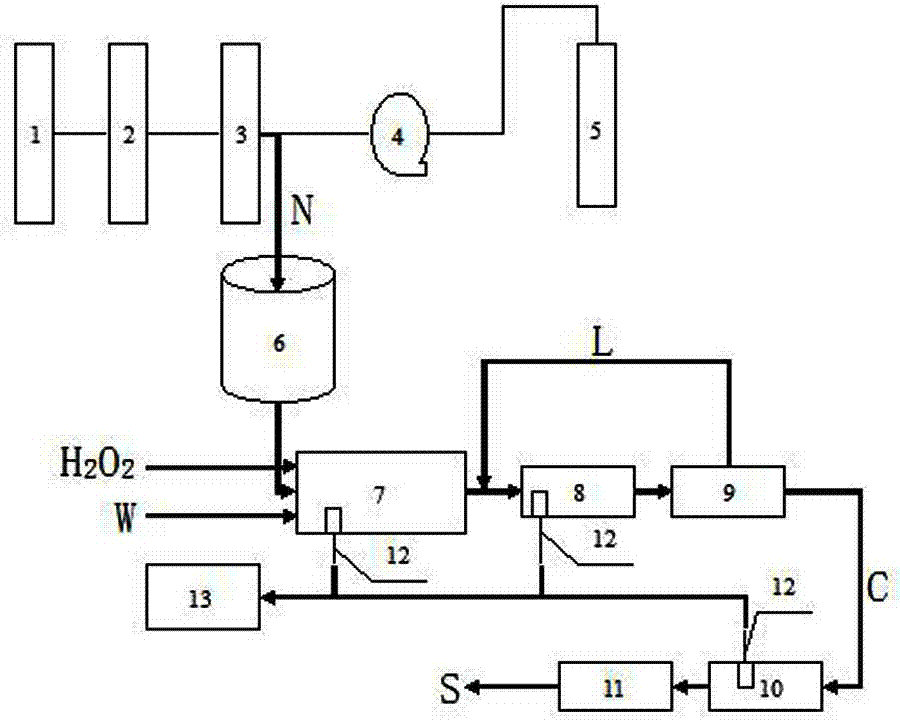
[0035] In this specific embodiment, the production method of urea peroxide is:
[0036](1) Lead the molten urine N evaporated and purified by the second-stage evaporation system 3 in the urea synthesis unit to the dissolving tank 6, and prepare a saturated urea solution with the water in the dissolving tank 6, and set the temperature of the material in the dissolving tank 6 Control at 40-50°C;
[0037] (2) Add the saturated urea solution prepared in step (1) into the tubular reactor 7 through a metering pump, and add 30% hydrogen peroxide aqueous solution to the tubular reactor 7 at the same time to ensure that urea and hydrogen peroxide H 2 o 2 The molar ratio of the urea is 1:1.1, and then a stabilizer W with 1% of the urea mass is added to the tubular reactor 7. The stabilizer W is formed by uniformly mixing potassium dihydrogen phosphate and polyethylene glycol with a mass ratio of 1:1. The above-mentioned materials were reacted in the tubular reactor 7 for 45 minutes, a...
Embodiment 2
[0042] In this specific embodiment, the production method of urea peroxide is:
[0043] (1) Lead the molten urine N evaporated and purified by the second-stage evaporation system 3 in the urea synthesis unit to the dissolving tank 6, and prepare a saturated urea solution with the water in the dissolving tank 6, and set the temperature of the material in the dissolving tank 6 Control at 40-50°C;
[0044] (2) Add the saturated urea solution prepared in step (1) into the tubular reactor 7 through a metering pump, and add 50% hydrogen peroxide aqueous solution to the tubular reactor 7 at the same time to ensure that urea and hydrogen peroxide H 2 o 2 The molar ratio of urea is 1:1.3, then add 1.6% stabilizer W of urea mass in the tubular reactor 7, the stabilizer W is formed by uniformly mixing potassium dihydrogen phosphate and polyethylene glycol with a mass ratio of 1:1 The above-mentioned materials were reacted in the tubular reactor 7 for 35 minutes, and the controlled reac...
Embodiment 3
[0049] In this specific embodiment, the production method of urea peroxide is:
[0050] (1) Lead the molten urine N evaporated and purified by the second-stage evaporation system 3 in the urea synthesis unit to the dissolving tank 6, and prepare a saturated urea solution with the water in the dissolving tank 6, and set the temperature of the material in the dissolving tank 6 Control at 40-50°C;
[0051] (2) Add the saturated urea solution prepared in step (1) into the tubular reactor 7 through a metering pump, and add 70% hydrogen peroxide aqueous solution to the tubular reactor 7 at the same time to ensure that urea and hydrogen peroxide H 2 o 2 The molar ratio of urea is 1:1.5, then add 2% stabilizer W of urea mass in the tubular reactor 7, the stabilizer W is formed by uniformly mixing potassium dihydrogen phosphate and polyethylene glycol with a mass ratio of 1:1 The above-mentioned materials were reacted in the tubular reactor 7 for 30 minutes, and the controlled reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com