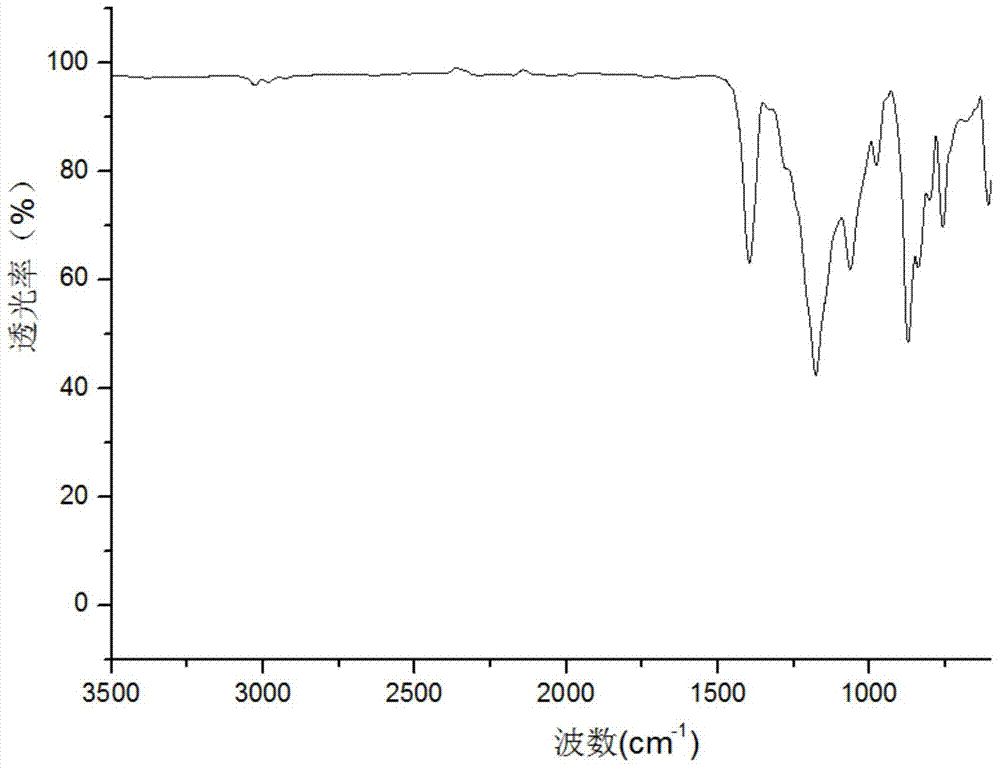
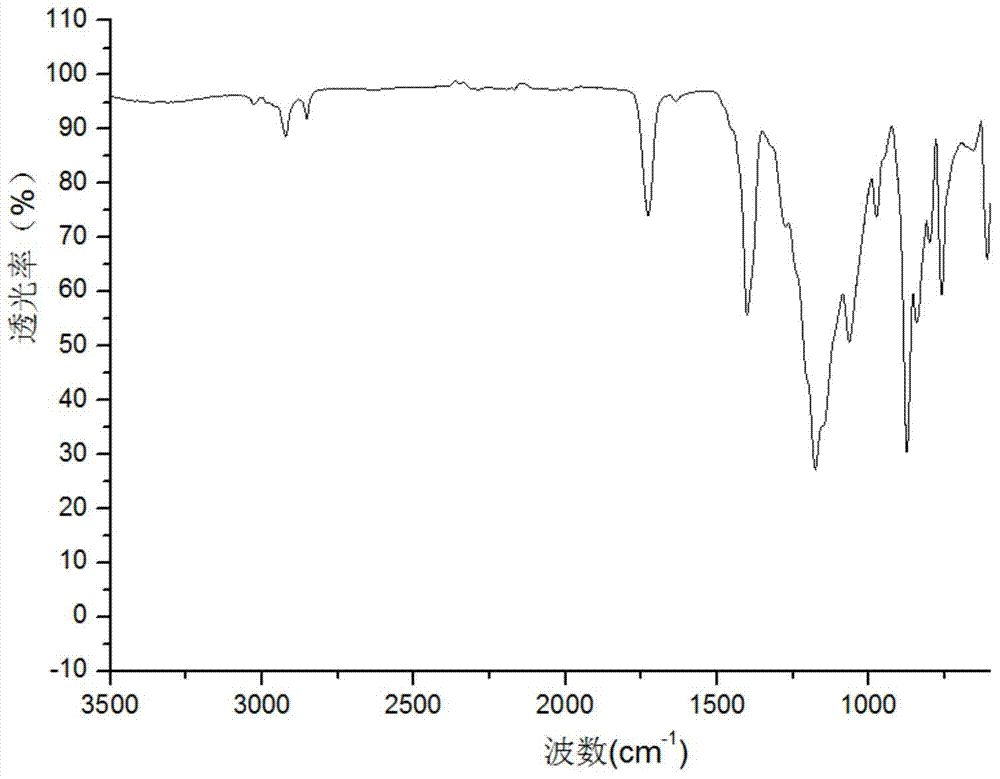
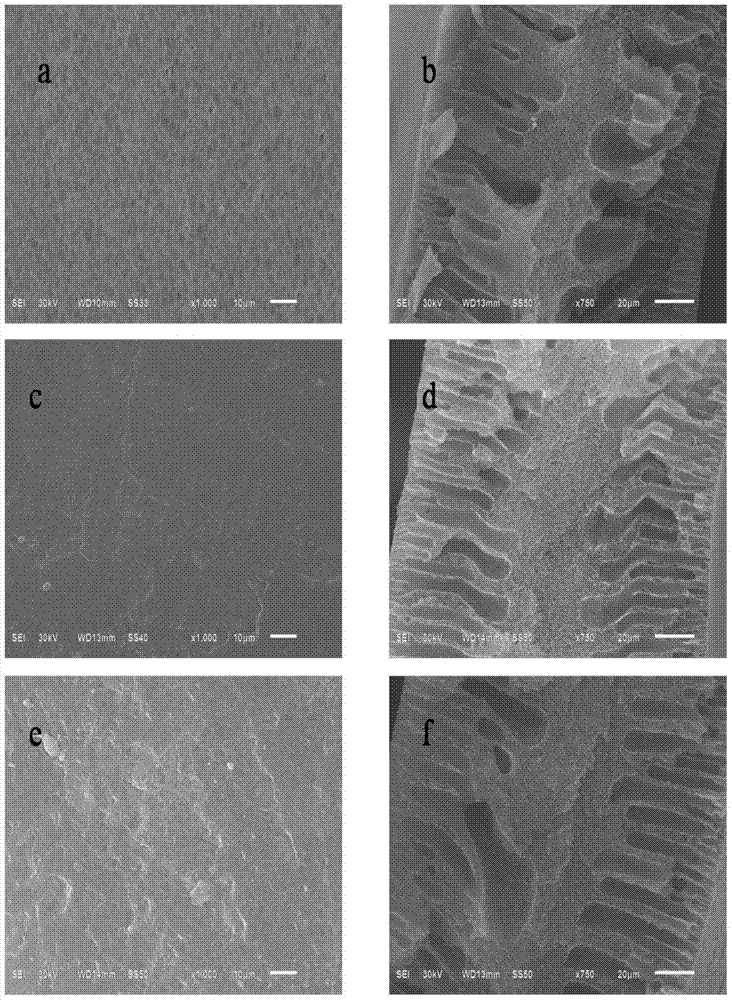
Preparation method for chiral separation benzenesulfonic acid amlodipine molecularly imprinted membrane
A technology of amlodipine besylate and ammonium chloride besylate, which is applied in the field of preparation of chiral separation amlodipine besylate molecularly imprinted membrane, can solve problems such as difficult industrial production, and achieve simple preparation method and good adsorption and separation function, the effect of low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The specific steps of the preparation method of the chiral separation amlodipine besylate molecularly imprinted membrane of the present invention are as follows:
[0028] (1) Prepare a NaOH aqueous solution with a concentration of 0.5mol / L, then put the PVDF hollow fiber membrane into it, soak it at 40°C for 3 hours, take out the residual alkali solution and dry it naturally, and soak the membrane in anhydrous methanol for later use;
[0029] (2) The template molecule S-amlodipine besylate is dissolved in chloroform, the molar volume ratio of S-amlodipine besylate and chloroform is 2:10 (mmol:mL), and then ultrasonically The template molecule is completely dissolved, add the functional monomer 4-vinylpyridine, the molar ratio of 4-vinylpyridine to S-amlodipine besylate is 5:2, leave it at room temperature for 2h, then add the cross-linking agent ethylene glycol Dimethacrylate and initiator azobisisobutyronitrile, the molar ratio of ethylene glycol dimethacrylate and azo...
Embodiment 2
[0032] The specific steps of the preparation method of the chiral separation amlodipine besylate molecularly imprinted membrane of the present invention are as follows:
[0033] (1) Prepare a NaOH aqueous solution with a concentration of 2mol / L, then put the PVDF hollow fiber membrane into it, soak it at 60°C for 1 hour, take out the residual alkali solution and dry it naturally, and soak the membrane in anhydrous methanol for use;
[0034] (2) The template molecule S-amlodipine besylate is dissolved in chloroform, the molar volume ratio of S-amlodipine besylate and chloroform is 2:30 (mmol:mL), and then ultrasonically The template molecules are completely dissolved, add the functional monomer 4-vinylpyridine, the molar ratio of 4-vinylpyridine to S-amlodipine besylate is 10:2, leave it at room temperature for 6h, and then add the cross-linking agent ethylene glycol Dimethacrylate and initiator azobisisobutyronitrile, the molar ratio of ethylene glycol dimethacrylate and azobi...
Embodiment 3
[0037] (1) Surface pretreatment of PVDF membrane: configure 1.0mol / L NaOH aqueous solution, put the PVDF hollow fiber membrane into it and soak it at 50°C for 2 hours, take out the residual alkali solution and dry it naturally, and soak the membrane in anhydrous methanol stand-by;
[0038] (2) Dissolve 2 mmol of template molecule S-amlodipine besylate in 20 mL of chloroform, sonicate for 5 minutes to completely dissolve the template molecule, add 8 mmol of functional monomer 4-vinylpyridine, and place at room temperature for 4 hours. After the functional monomers and template molecules are sufficiently pre-polymerized, add 40 mmol of ethylene glycol dimethacrylate as a crosslinking agent and 0.88 mmol of azobisisobutyronitrile as an initiator, and mix them uniformly by ultrasonic vibration for 5 minutes to obtain a pre-polymerization solution.
[0039] (3) The PVDF hollow fiber membrane treated above was immersed in the pre-polymerization solution for 5 minutes, taken out to d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com