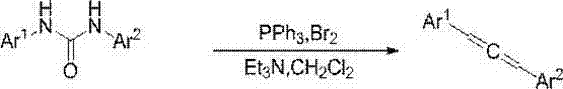
Synthesis method of disubstituted urea compounds
A technology of a disubstituted urea and a synthesis method, which is applied in the field of synthesis of urea compounds, can solve the problems of danger, large method limitations, expensive catalysts and the like, and achieves the effects of cheap catalysts, good development prospects and excellent yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: N-phenyl-N'-(2-pyridyl)urea
[0031] N-phenyl-N'-(2-pyridyl)urea adopts the following steps: ①Add 25.7 grams of p-nitrophenylacetyl 2-pyridinamide, 10 milliliters of aniline, and 1.9 grams of cuprous iodide in a 250 milliliter reaction kettle , 3.6 g of 1,10-phenanthroline, 200 ml of N,N-dimethylformamide, heated to 120°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, add excess saturated saline to the system, extract with ethyl acetate, combine the organic phases, wash the organic phases with saturated saline, and dry the organic phases After removing the solvent, the crude product was obtained; ③ the crude product was purified by column chromatography gradient elution (petroleum ether: ethyl acetate = 1:5 to 1:3) to obtain N-phenyl-N'-(2-pyridyl ) urea 15.8 grams, productive rate is 74%.
[0032] δ 10.47 (s, 1H), 9.40 (s, 1H), 8.25-8.22 (m, 1H), 7.72-7.69(m, 1H), 7.49-7.46 (m, 3H), ...
Embodiment 2
[0035] Example 2: N-(4-bromophenyl)-N'-(2-pyridyl)urea
[0036]N-(4-bromophenyl)-N'-(2-pyridyl)urea adopts the following steps: 1. add 25.7 grams of p-nitrophenylacetyl 2-pyridinamide, 4-bromoaniline 25 gram, 1.9 grams of cuprous iodide, 3.6 grams of 1,10-phenanthroline, 200 milliliters of N,N-dimethylformamide, heated to 120°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, add excess saturated saline to the system, extract with ethyl acetate, combine the organic phases, wash the organic phases with saturated saline, and dry the organic phases After removing the solvent, the crude product was obtained; ③ the crude product was purified by column chromatography (petroleum ether: ethyl acetate = 1:5) to obtain N-(4-bromophenyl)-N'-(2-pyridyl)urea 17.5 g, 60% yield.
[0037] δ 10.65 (s, 1H), 9.50 (s, 1H), 8.27 (td, J = 7.5, 2.0 Hz, 1H), 7.55-7.45(m, 5H);
[0038] 13 C NMR (DMSO-d 6 , 125 MHz): δ 153...
Embodiment 3
[0040] Example three: N-(4-chlorophenyl)-N'-(2-pyridyl)urea
[0041] N-(4-chlorophenyl)-N'-(2-pyridyl)urea adopts the following steps: 1. Add 25.7 grams of p-nitrophenylacetyl 2-pyridinamide, 4-chloroaniline 25.4 ml, 3.8 g of cuprous iodide, 7.2 g of 1,10-phenanthroline, 200 ml of N,N-dimethylformamide, heated to 110°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, add excess saturated saline to the system, extract with ethyl acetate, combine the organic phases, wash the organic phases with saturated saline, and dry the organic phases After removing the solvent, the crude product was obtained; ③ the crude product was purified by column chromatography (petroleum ether: ethyl acetate = 1:5) to obtain N-(4-chlorophenyl)-N'-(2-pyridyl)urea 15.3 g, 62% yield.
[0042] δ 10.65 (s, 1H), 9.49 (s, 1H), 8.29-8.26 (m, 1H), 7.77-7.73(m, 1H), 7.58-7.54 (m, 2H), 7.47 (d, J = 8.0 Hz,1H), 7.39-7.33 (m, 2H),7.04-6.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com