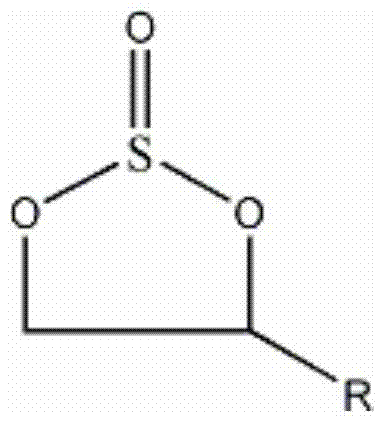
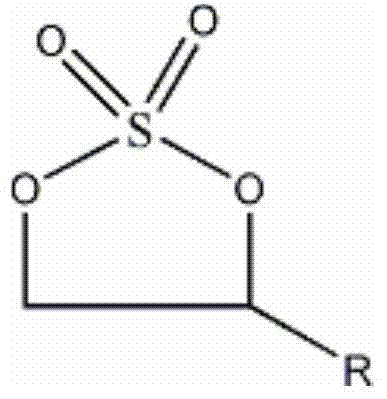
Method for preparing cyclic sulphate
A technology of cyclic sulfate and cyclic sulfite, which is applied in the field of preparation of cyclic sulfate, can solve the problems of high process cost, low yield, and many side reactions, and achieve high yield and purity, and easy raw materials Easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 54g (0.5mol) of vinyl sulfite, 1620g of dichloromethane, 14.6g of rhenium trichloride, and 250g of sodium bicarbonate to the reaction flask in sequence, stir and cool the reaction system to 10°C, and then adjust the mass percent concentration to 20°C. 850 g of aqueous potassium hydrogen persulfate solution was added dropwise to the reaction system within 2 hours. Stir vigorously for 60 min after the dropwise addition, separate the organic layer after filtration, and remove the organic solvent to obtain a crude vinyl sulfate product with a purity of 98.6%. The crude product was recrystallized three times with dichloromethane to obtain vinyl sulfate with a purity of 99.7% and a yield of 86.6%.
Embodiment 2
[0027] Add 4-methylvinylsulfite 61g (0.5mol), acetonitrile 100g, ethylene glycol dimethyl ether 100g, toluene 776g, ruthenium trichloride hydrate 2.6g successively in the reaction flask, stir and adjust the reaction system to 50 DEG C, then the solution that the potassium persulfate compound salt 84.5g that contains potassium hydrogen persulfate 45% (mass percentage concentration) and 300g water is added dropwise in the kettle liquid in 1 hour, divides more Add 30 g of potassium carbonate once to control the pH of the system to 7-8. Continue vigorously stirring for 600 min after the dropwise addition, separate the organic layer after filtration, and remove the organic solvent from the organic layer to obtain crude 4-methyl vinyl sulfate with a purity of 97.6%. The crude product was rectified under reduced pressure to obtain 4-methyl vinyl sulfate with a purity of 99.1% and a yield of 85.3%.
Embodiment 3
[0029] Add 99.1 g (0.5 mol) of 4-benzyl vinyl sulfite, 18.2 g of N,N-dimethylimidazolinone, 180 g of chlorobenzene, 200 g of disodium hydrogen phosphate, and 0.04 g of manganese sulfate into the reaction flask in sequence. Under stirring, the temperature of the reaction system was adjusted to 25° C., and then the solution made of potassium persulfate compound salt 1690 g and 5000 g water containing potassium hydrogen persulfate 45% (mass percentage concentration) was added dropwise in the still liquid within 2 hours. Continue to stir vigorously for 300 min after the dropwise addition, separate the organic layer after filtration, and then remove the organic solvent by rotary evaporation to obtain the crude product of 4-benzyl vinyl sulfate with a purity of 95.7%. The crude product is refined once with toluene to obtain the refined product 4-benzene Vinyl methyl sulfate, purity 95.7%, yield 84.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com