Angucycline compounds and application of angucycline compounds in preparation of anti-tumour or antibacterial medicine
A technology of antitumor drugs and antibacterial drugs, applied in the field of natural products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Where compound 1:
[0029] Compound 2:
[0030] Compound 3:
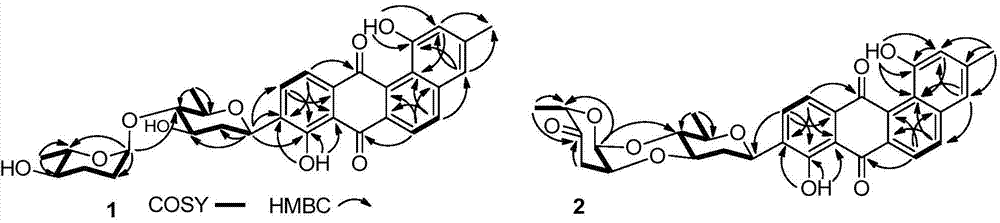
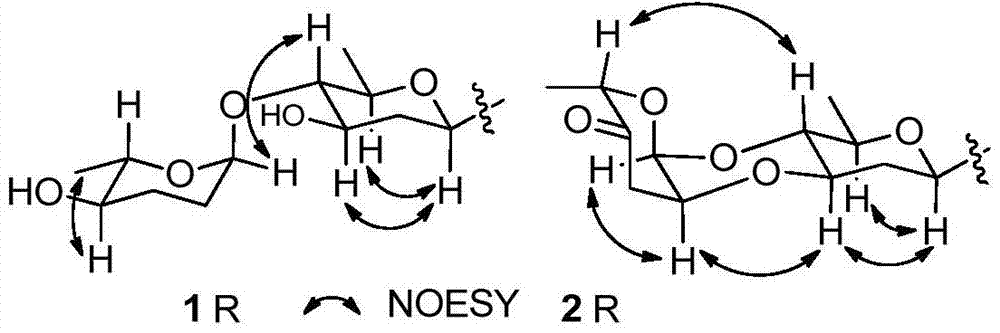
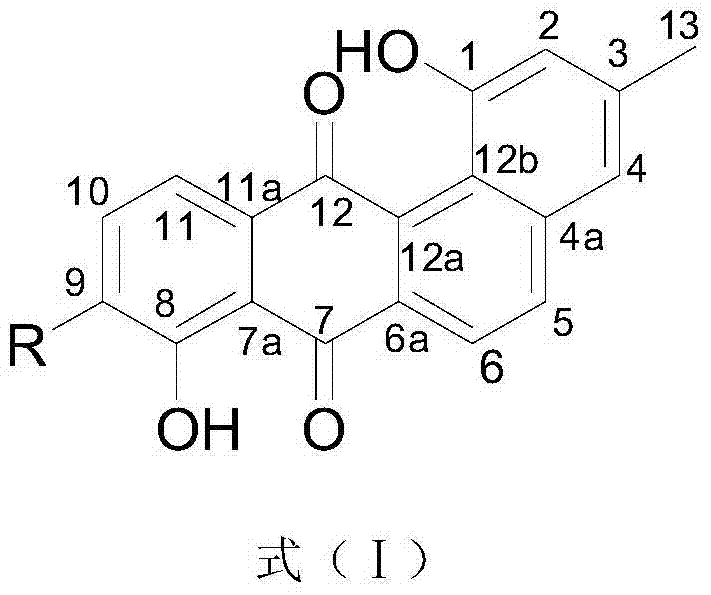
[0031] Preparation and structure identification of compound 1 and compound 2 as shown in formula (I), and compound 3
[0032] 1. Preparation of compound 1, compound 2 or compound 3 as shown in formula (I)
[0033] 1. Seed culture
[0034] (1) Seed medium (MAM2ab formula): by mass fraction, including 0.5% soluble starch, 0.5% soybean flour, 2% glucose, 0.2% yeast extract, 0.2% peptone, K 2 HPO 4 0.05%, MgSO 4 ·7H 2 O 0.05%, NaCl 0.4%, Coarse Sea Salt 0.3%, CaCO 3 0.2%, the balance is water, pH 7.2-7.4. According to the formula, mix all the components and pack them into 250mL Erlenmeyer flasks, fill each bottle with 50mL, sterilize at 121°C for 20 minutes, and use it as a spare seed medium. The fermentation medium is the same as the seed medium. .
[0035] (2) Cultivation of seeds: insert the mycelia or spores of the strain Streptomyces rubrogriseus SCSIO 11594 into the seed medium (...
Embodiment 2
[0054] To the experiment of the antitumor cell of the naphthoquinone sesquiterpenes of embodiment 1-compound 1, compound 2 and compound 3
[0055] International tumor cell lines are used, including lung cancer cell line (A549), nasopharyngeal carcinoma cell line (CNE2), breast cancer cell line (MCF-7), and human liver cancer cell line (HepG-2). The test method is the internationally accepted SRB method, with cisplatin as the positive control and normal hepatocytes (HL7702) as the reference. Three parallel experiments were performed for each cell line. The experimental results are shown in Table 2:
[0056] Table 2 compounds 1, 2 and 3 inhibit tumor cell lines (IC 50 ,μM)
[0057]
[0058] The above experimental results show that compound 2 has significant inhibitory activity on the four tested tumor cell lines, and its inhibitory activity on the tested tumor cell lines is an order of magnitude stronger than that of the positive control drug cisplatin, and its inhibitory ac...
Embodiment 3
[0060] Bacteriostasis experiments on the keratin compounds of Example 1-compound 1, compound 2 and compound 3.
[0061] Escherichia coli ATCC 25922, Staphyloccocus aureus ATCC29213, Enterococcus faecalis ATCC29212, Micrococcus luteus, Methicillin-resistant Staphylococcus aureus (MREA) and Methicillin-resistant Staphylococcus epidermidis (MRSE shhs-E1) was used as the test bacterium, and 100 μl of the compound 1-3 was used to test the activity of the system according to the microplate method of CLSI. Specifically:
[0062] 1) Bacterial culture. Cultivate the experimental bacteria with Mueller-Hinton (MH) broth medium, when it grows for 8-12h to about 0.5 Mcfarland concentration (1×10 8 CFU) for backup. And configure a certain concentration of the sample solution. Concentrations of samples and positive controls were configured, and positive controls were selected from ampicillin and kanamycin (water soluble).
[0063] 2) Prepare samples and dilute bacteria solution. The sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com