Polypeptide as well as phlogenzym tablet composition and application thereof
A composition and drug technology, applied in the field of preparation of the above chemical products, can solve the problems of changing the properties of active proteins, reducing the activity of proteins, increasing potential safety hazards, etc., and achieving the effects of convenient equipment, good stability, and easy popularization and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 polypeptide of the present invention
[0025] After long-term research, the inventors have developed the polypeptide of the present invention and its optimized expression gene sequence in yeast, wherein the nucleotide of the gene is shown in SEQ ID NO: 1, and the amino acid of the polypeptide of the present invention encoded by it is The sequence is shown in SEQ ID NO:2. We commissioned Xi'an Xintong Pharmaceutical Research Co., Ltd. to synthesize the gene and clone it into yeast cells. In short, use the upstream and downstream primers shown in SEQ ID NO: 3 and 4 to perform PCR amplification using the synthesized gene as a template , the amplified product was digested by EcoR I and Xba I and connected between the multiple cloning sites of EcoR I and Xba I of pPICZαA (available from Invitrogen). After sequencing and confirming that it was correct, the positive plasmid was transformed into yeast In the GS115 strain (available from Invitrog...
Embodiment 2
[0028] Embodiment 2 Stability study of the polypeptide of the present invention
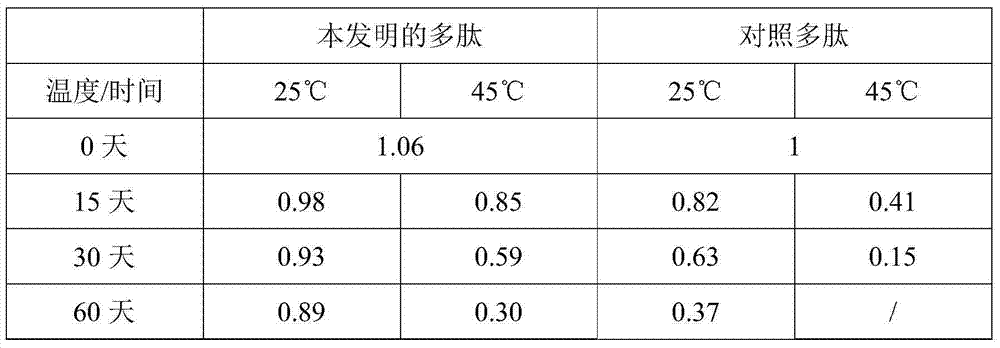
[0029] With reference to the protease activity detection method described in Harrach et al. (J Protein Chem, 14:41-52), the polypeptide of the present invention prepared in Example 1 and the reference polypeptide prepared in high temperature storage for different times were respectively detected against the substrate L-Pyr-Phe- Hydrolytic activity of Leu-pNA. For the convenience of comparison, the activity of 1 mg of the control polypeptide stored at high temperature for 0 days (that is, taken out from the storage state at -20°C for detection immediately) was counted as 1, and the relative activities of 1 mg of different polypeptides stored under different conditions are shown in Table 1. shown.
[0030] The stability of table 1 polypeptide
[0031]
[0032] As can be seen from Table 1, the activity of an equivalent amount of polypeptide of the present invention and the wild-type control pol...
Embodiment 3 2
[0033] The preparation of embodiment 3 two enzyme tablets
[0034] Take 502 grams of starch and 130 grams of lactose, add 50% (V / V) ethanol to make a soft material, granulate, dry at 45°C until the water content is less than 5% (W / W), pass through a 20-mesh sieve, That is, the ball core is obtained.
[0035] Get 90 grams of the polypeptide of the present invention prepared in Example 1, 48 grams of trypsin and 100 grams of rutin, mix evenly with 5 grams of silicon dioxide micropowder, 25 grams of microcrystalline cellulose and the above-mentioned ball core, press into tablets, and press into 1000 piece.
[0036] Get 160 grams of oprdry standard coating powder, dissolve in 4L of 75% (V / V) ethanol, and prepare enteric coating solution. The above-mentioned formed tablets are placed in the fluidized bed, start the fluidized bed, and spray the enteric coating solution to these formed tablets with atomizing nozzles, wherein the parameters of the fluidized bed setting are: bed temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com