Porous metallic cathode material doped with lithium manganate/carbon for composite lithium batteries, and preparation method of porous metallic cathode material
A technology of heteromanganate lithium and porous metal, which is applied in the field of preparation of new porous metal-doped lithium manganate/carbon composite lithium battery cathode materials, can solve the problems that the sintering temperature cannot be too high and the grain crystallinity is not ideal. Achieve good discharge specific capacity, good crystallinity, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
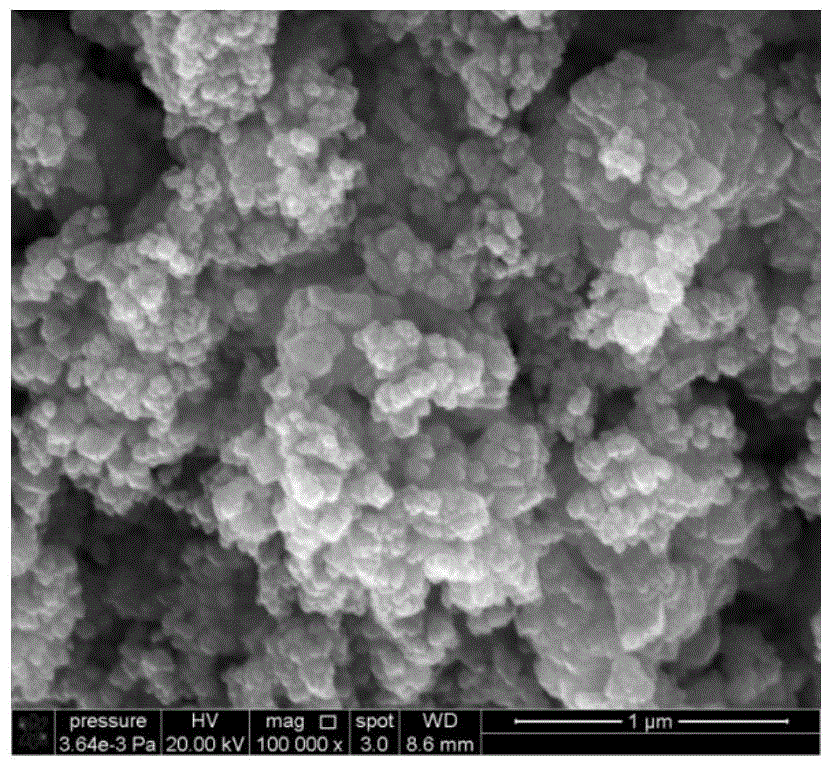
Image
Examples
Embodiment 1
[0024] In the first step, the polymer p123 with a molar mass of 5800 was dissolved in an appropriate amount of absolute ethanol, and stirred continuously at room temperature to obtain 20 mL of P123 gel with a uniform dispersion concentration of 0.05 g / mL.
[0025] In the second step, solid Mn(NO 3 ) 2 4H 2 O, LiNO 3 , Fe(NO 3 ) 3 9H 2 O is added in a molar ratio of 9:5:1, LiNO 3 The amount is 0.05mol.
[0026] In the third step, the above mixture was magnetically stirred at a medium speed for 12 h at room temperature.
[0027] In the fourth step, the uniformly mixed sol is air-dried at 60° C. for 24 hours in an air-blast drying oven.
[0028] In the fifth step, the dried mixture is heated in a muffle furnace at 2° C. / min to 500° C. in an air atmosphere, and kept for 2 hours.
[0029] In the sixth step, the prepared sample was uniformly dispersed in a mixed solution of 1.2 g of anhydrous glucose dissolved in 5 mL of deionized water and 15 mL of absolute ethanol, magnet...
Embodiment 2
[0034] In the first step, the polymer p123 with a molar mass of 5800 was dissolved in an appropriate amount of absolute ethanol, and stirred continuously at room temperature to obtain 20 mL of P123 gel with a uniform dispersion concentration of 0.08 g / mL.
[0035] In the second step, solid Mn(NO 3 ) 2 4H 2 O, LiNO 3 , Fe(NO 3 ) 3 9H 2 O is added in a molar ratio of 9:5:1, LiNO 3 The amount is 0.1mol.
[0036] In the third step, the above mixture was magnetically stirred at a medium speed for 16 h at room temperature.
[0037] In the fourth step, the uniformly mixed sol was air-dried at 70° C. for 36 hours in an air-blast drying oven.
[0038] In the fifth step, the dried mixture is heated in a muffle furnace at 3° C. / min to 600° C. in an air atmosphere, and kept for 3 hours.
[0039] In the sixth step, the prepared sample was uniformly dispersed in a mixed solution of 1.4 g of anhydrous glucose dissolved in 5 mL of deionized water and 15 mL of absolute ethanol, magnet...
Embodiment 3
[0044] In the first step, the polymer p123 with a molar mass of 5800 was dissolved in an appropriate amount of absolute ethanol, and stirred continuously at room temperature to obtain 20 mL of P123 gel with a uniform dispersion concentration of 0.1 g / mL.
[0045] In the second step, solid Mn(NO 3 ) 2 4H 2 O, LiNO 3 , Fe(NO 3 ) 3 9H 2 O is added in a molar ratio of 9:5:1, LiNO 3 The amount is 0.5mol.
[0046] In the third step, the above mixture was magnetically stirred at a medium speed for 24 h at room temperature.
[0047] In the fourth step, the uniformly mixed sol is air-dried at 80° C. for 48 hours in an air-blast drying oven.
[0048] In the fifth step, the dried mixture is heated up to 700° C. in a muffle furnace at 5° C. / min in an air atmosphere, and kept for 4 hours.
[0049] In the sixth step, the prepared sample was uniformly dispersed in a mixed solution of 1.6 g of anhydrous glucose dissolved in 5 mL of deionized water and 15 mL of absolute ethanol, magne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com