Icariin H2 crystal form, preparation method of icariin H2 crystal form, medicine composition and application
A technology of icariin and its composition, applied in the field of medicinal chemistry, can solve the problems of poor water solubility of icariin, poor oral absorption, and limitation of clinical application, and achieve good reproducibility, favorable absorption and utilization, and oral absorption high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take 30 mg of icariin in a bottle, add 5 mL of ethanol / water (volume ratio 1:1), dissolve it by ultrasonication, and dissolve it at 50°C for 1 hour, then filter it while it is hot to prevent it from becoming supersaturated, and then put it in Volatilize at 50°C, and the obtained solid is the crystal form of icariin H2.
Embodiment 2
[0037] Take 30mg of icariin in a bottle, add methanol / water (volume ratio 2:3) 3mL, place on a magnetic stirrer, suspend at room temperature for 3 days, discard the supernatant after centrifugation, and centrifuge The solid was evaporated to dryness to obtain icariin H2 crystal form.
Embodiment 3
[0039] Take 30 mg of icariin in a bottle, add 5 mL of dioxane / water (volume ratio 2:1) mixed solvent, ultrasonically dissolve it, and dissolve it at 50°C for 1 hour, then filter while it is hot to avoid excessive Saturated state, then volatilize at 50°C, the obtained solid is icariin H2 crystal form.
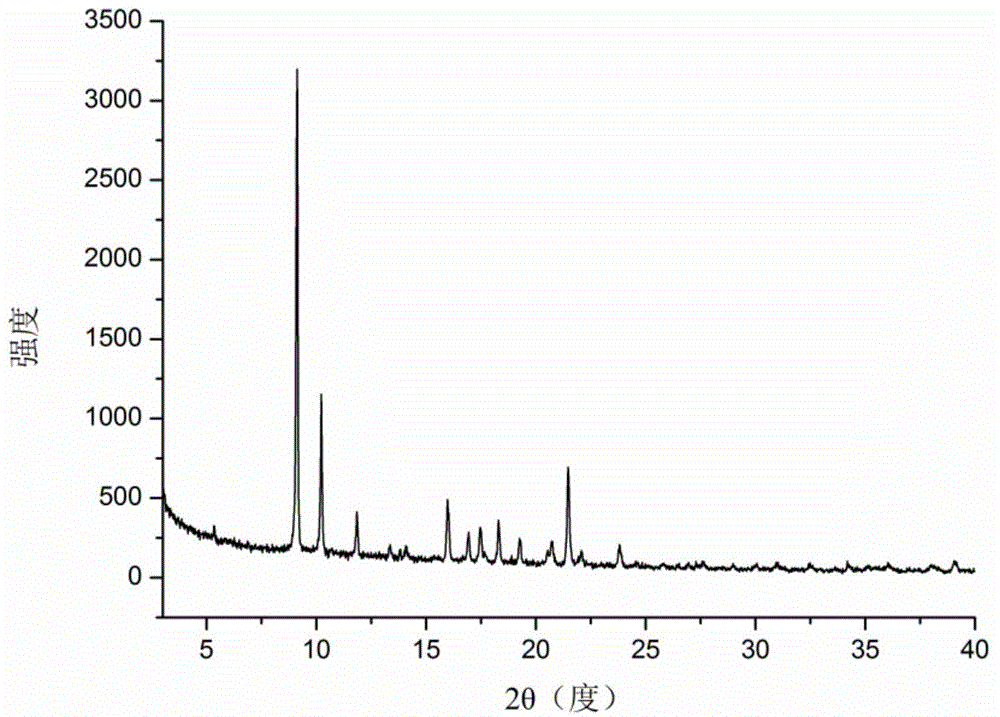
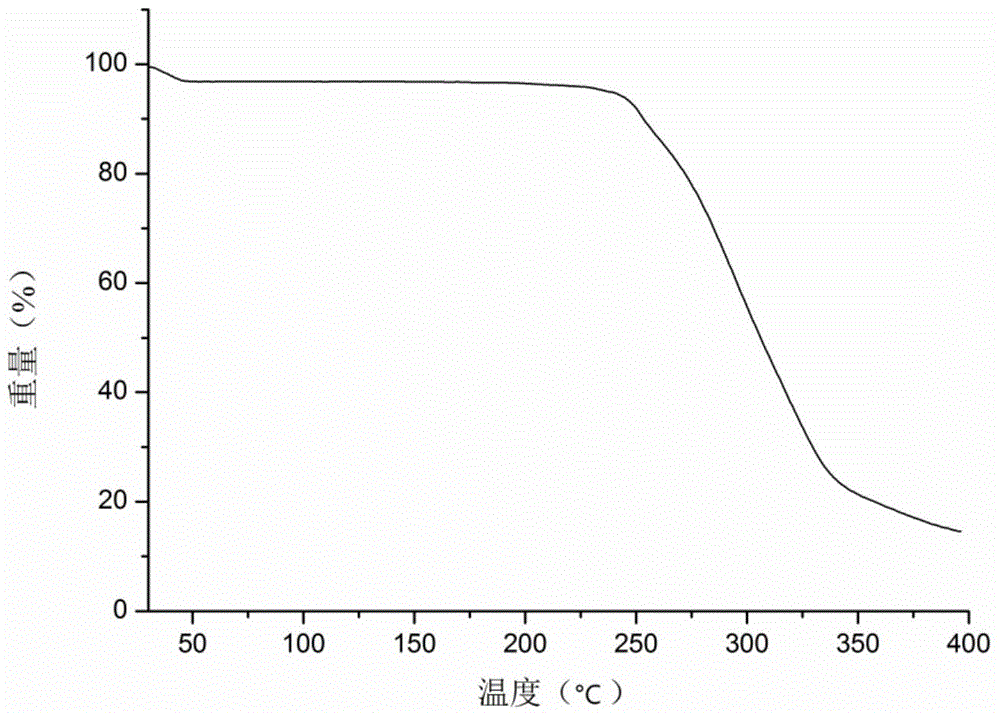
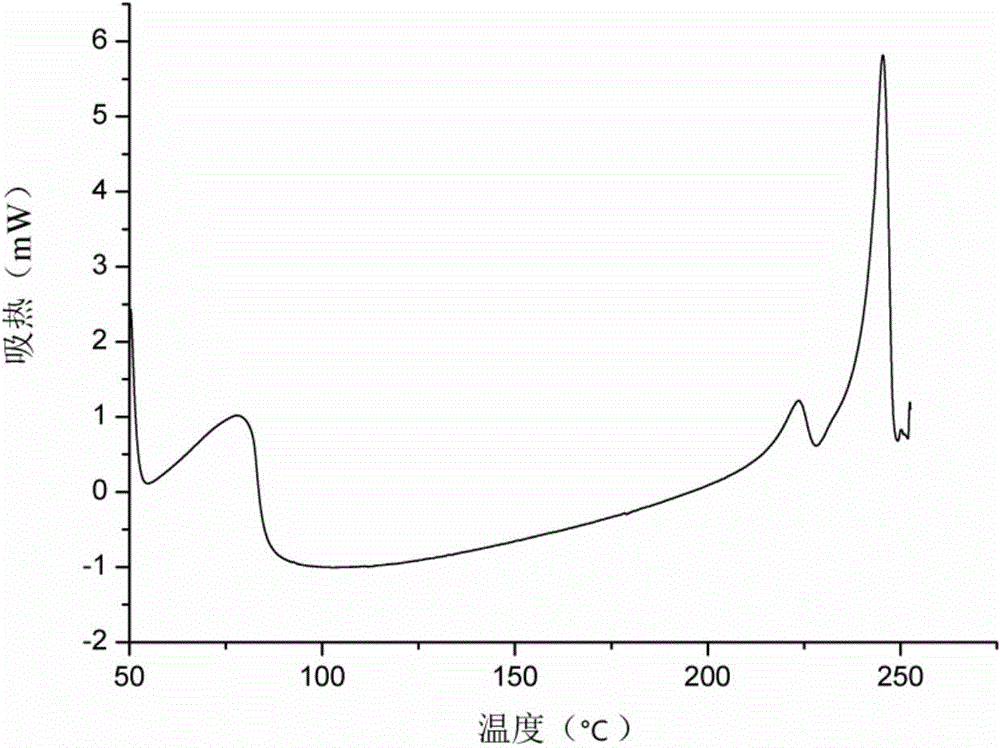
[0040] A kind of icariin H2 crystal form provided by the present invention, through X-ray powder diffraction (XRPD), thermogravimetric analysis (TG), differential scanning calorimetry (DSC), dynamic water adsorption (DVS), infrared ( IR) and Raman (Raman) and other solid-state methods for characterization.
[0041] Carry out X-ray powder diffraction analysis to the icariin H2 crystal form solid sample that embodiment 1 makes, it adopts the diffractometer of Bruker D8 advance type of German Bruker Instrument Co., Ltd., adopts Cu-Kα ray The voltage is 40 kV, the current is 40 mA, the scanning speed is 12 degrees per minute, the step size is 0.02 degrees, and each step takes 0.1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com