Human endothelial cell cadherins fusion protein, and preparation method and application thereof
A human endothelial cell and fusion protein technology, applied in the field of human endothelial cell-cadherin fusion protein and its preparation, can solve the problems of easy immune response, poor biocompatibility, poor mechanical properties, etc., and achieve simple and easy modification Easy to operate, easy to operate, stable process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Construction of pcDNA3.1 / VE-cad-Fc eukaryotic expression vector
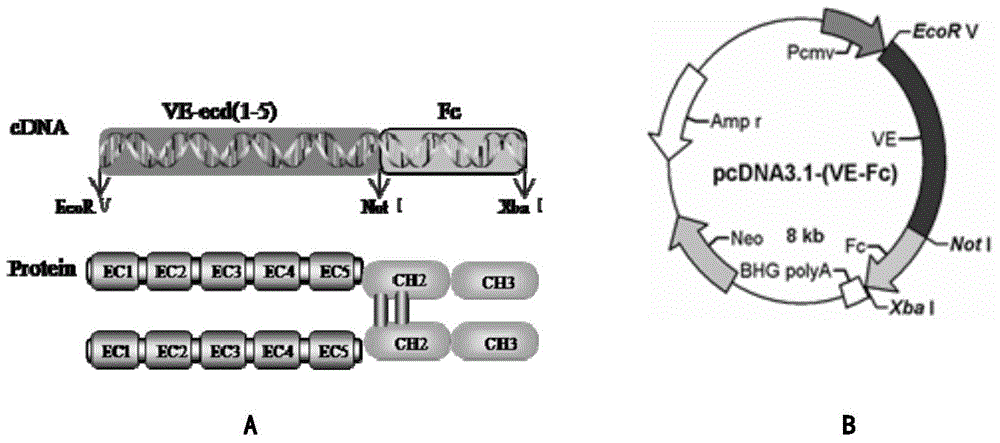
[0049] According to the human VE-cadherin protein sequence and functional partitions recorded in UniProt, the upstream primer (P1) was designed; 5′-CCGGATATCATGCAGAGGCTCATGATGCTCC-3′, the EcoR Ⅴ restriction site was introduced, and the downstream primer: (P2) 5′-AAGCGGCCGCTCTGGGCGGCCATATC-3′ , the introduction of Not I restriction site. Using synthetic primers to specifically amplify the hVE-cadherin extracellular domain gene, and sequence the DNA, see figure 1 a. 1% agarose gel electrophoresis to analyze the PCR product, the PCR product recovered from the gel was digested by EcoR V and Not I, and inserted between the pcDNA3.1-Fc EcoR V and Not I sites, namely pcDNA3.1 / VE -cadherin-Fc, plasmid pcDNA3.1-Fc was kindly provided by Professor Akaike Toshihiro, Tokyo Institute of Technology, see figure 1 b. Gene sequencing was detected by Nanjing GenScript. The full-length hVE-cad-Fc gene sequence is the se...
Embodiment 2
[0051] Expression and purification of hVE-cad-Fc fusion protein
[0052] The plasmid pcDNA3.1-VE-cadherin-Fc containing the target gene constructed in Example 1 was transfected into 293-6E cells by PEI and kept at 37°C in 5% CO 2 After suspension culture, the cell supernatant was collected after the sixth day, and the hVE-cad-Fc fusion protein was purified by rProtein A FF column. SDS-PAGE electrophoresis analysis showed that the molecular weight of the obtained product was consistent with the predicted theoretical value, see figure 2 a. It has been determined that 10 mg of the target protein can be obtained from 1 L of 293-6E cell culture medium with a purity of about 87%. The resulting fusion protein hVE-cad-Fc is bound to the surface of the hydrophobic material through the Fc end to form an endothelial cell cadherin fusion protein modified for endothelial cell culture, see figure 2 b.
Embodiment 3
[0054] Surface Modification of Hydrophobic Materials by hVE-cad-Fc Fusion Protein, i.e. Substrate Conditions and Its Evaluation
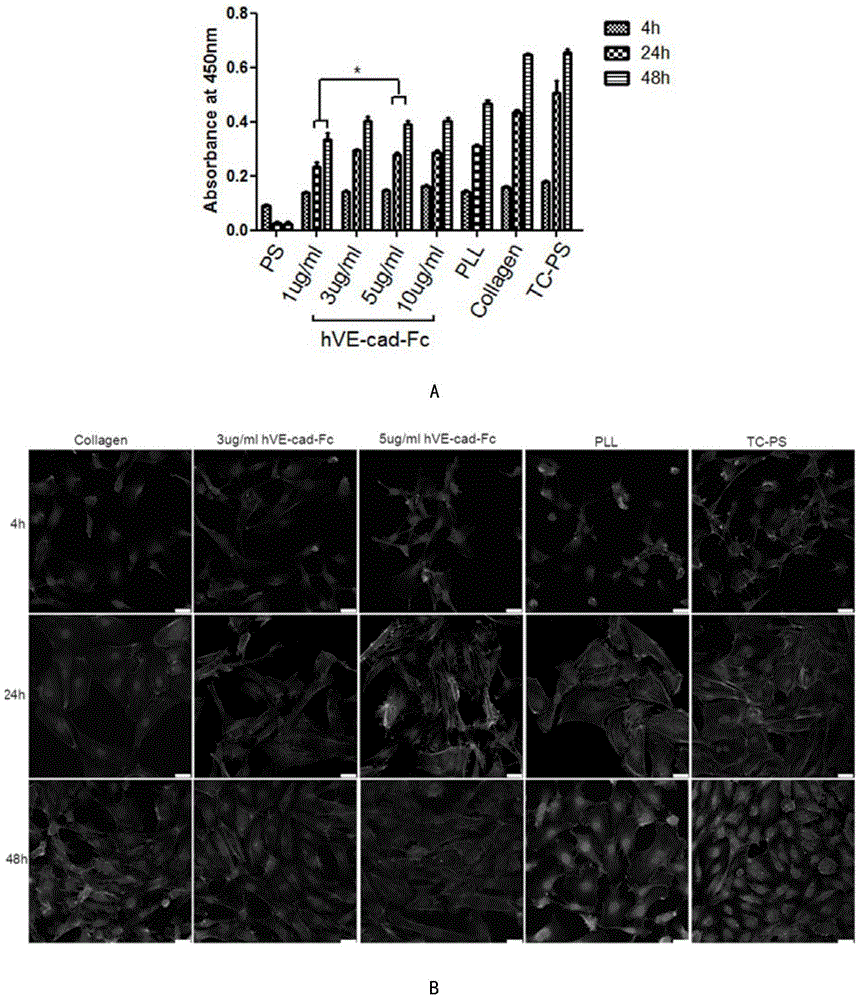
[0055] The maximum immobilization amount and long-term stability of the fusion protein were detected by Elisa method. Dissolve hVE-cad-Fc in PBS to prepare solutions of different concentrations, spread them on polystyrene 96-well plates, incubate at 37°C for 2 hours, wash with PBS for 3 times, then block with 1% BSA for 1 hour at room temperature, and add anti-hVE-cadherin antibody Incubate at 37°C for 1 hour, wash with PBS for 3 times, add horseradish peroxidase-labeled secondary antibody and incubate at 37° for half an hour, wash with PBS for 3 times, add TBA chromogenic solution, incubate at 37°C for 10 minutes in the dark, add stop solution within 10 minutes Microplate reader detection 450nm, see image 3 a. In the hVE-cad-Fc fusion protein stability experiment, the fusion protein incubation time is 1d, 3d, 5d, 7d, the concentration is 10ug / ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com