A kind of surface cyanide modified nano-metal material and preparation method thereof
A technology of nanomaterials, nanometals, applied in the field of chemistry, which can solve problems such as environmental and human health threats
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
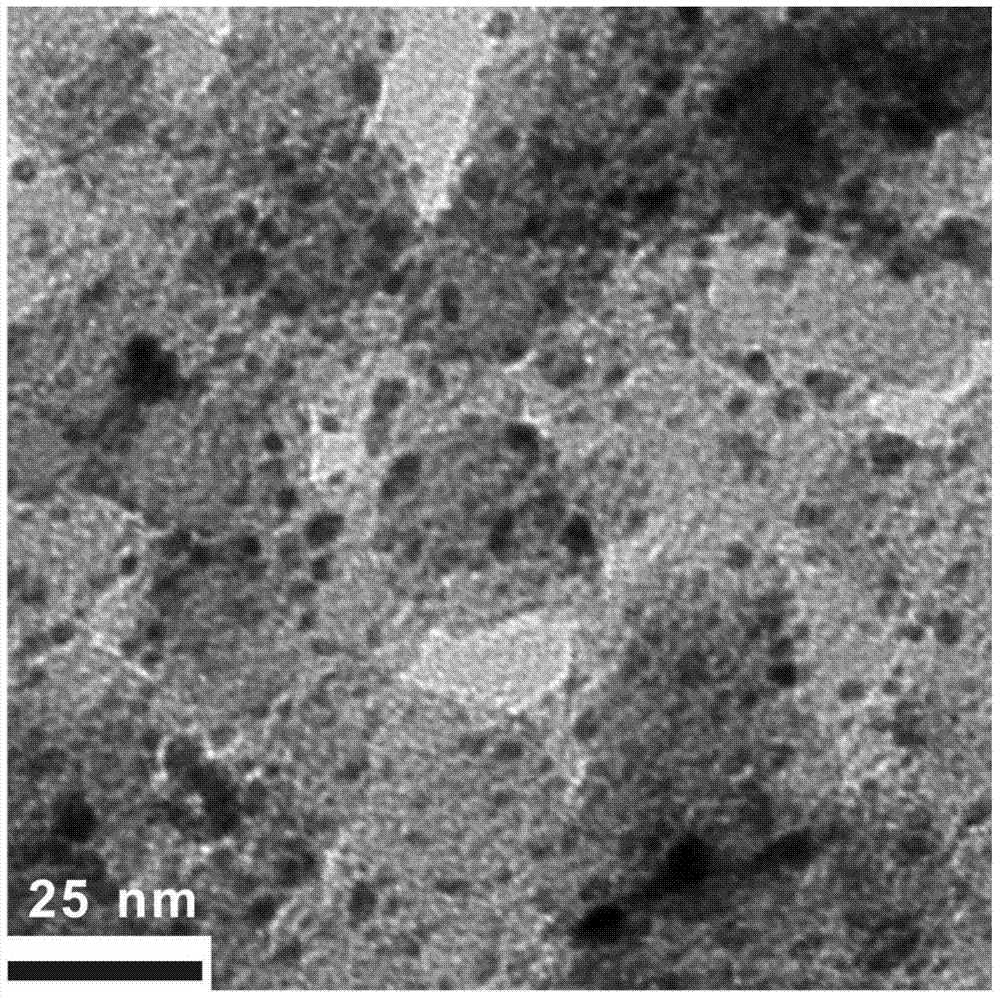
[0057] Mix 1.2 mg of nano-platinum-supported activated carbon material, 2 mL of acetonitrile, and 0.6 mmol of hydrogen peroxide, and stir at 25°C for 30 minutes under ultraviolet light, and for 5 hours without ultraviolet light; the product is centrifuged and dried to obtain surface cyanide. modified Pt / C catalysts. figure 1 It is a transmission electron microscope photo of the surface of the Pt / C catalyst modified by cyano groups. It can be seen in the figure that the platinum particles modified by this method are nanoparticles, uniformly dispersed, and the particle size is 1.5-3.5 nanometers. According to the XPS element energy calculation, the coordination ratio between the ligand (CN) and the metal Pt is 1:1, and 30% of the metal atoms on the surface of the nano-metal Pt are coordinated with CN.
Embodiment 2
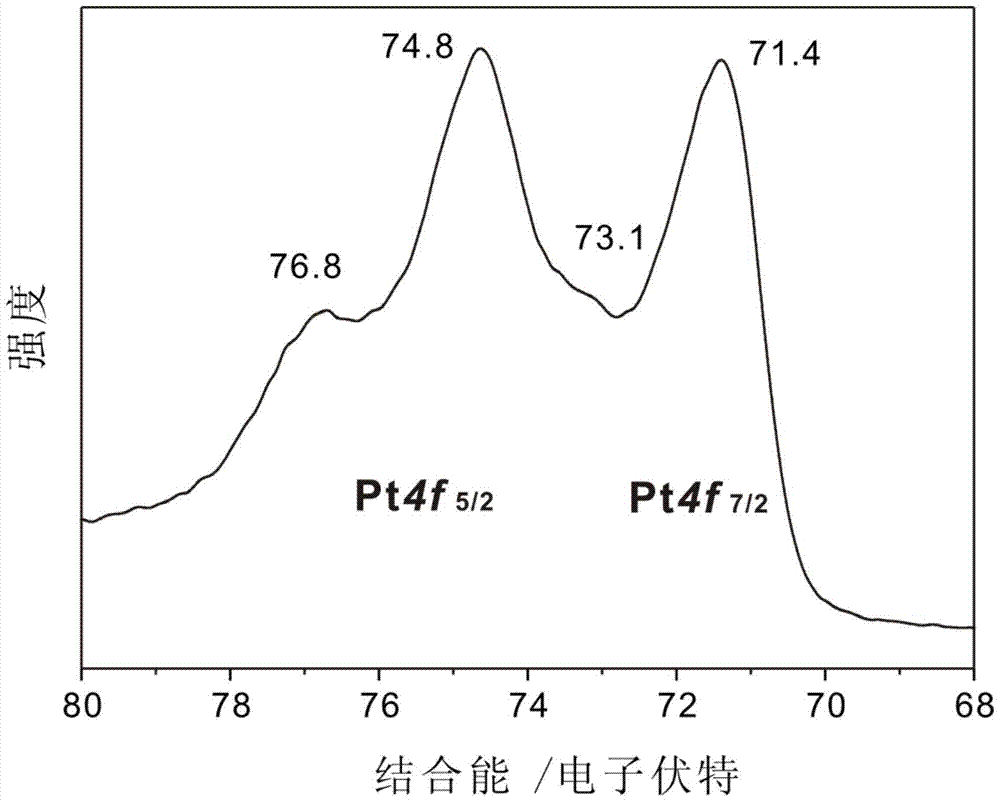
[0061] Mix 5.8 mg of nano-platinum-loaded activated carbon material, 3 mL of acetonitrile, and 1.2 mmol of hydrogen peroxide, and stir at 25°C for 20 minutes under ultraviolet light, and for 20 hours without ultraviolet light; the product is centrifuged and dried to obtain surface cyanide. modified Pt / C catalysts. figure 2 is the XPS spectrum of the Pt / C catalyst modified by surface cyano groups, showing that Pt 4f 5 / 2 and Pt 4f 7 / 2 The peak positions correspond not only to Pt(0), but also to Pt(I) and Pt(II). According to the XPS element energy calculation, the coordination ratio between the ligand (CN) and the metal Pt is 1:1, and 50% of the metal atoms on the surface of the nano-metal Pt are coordinated with CN.
Embodiment 3
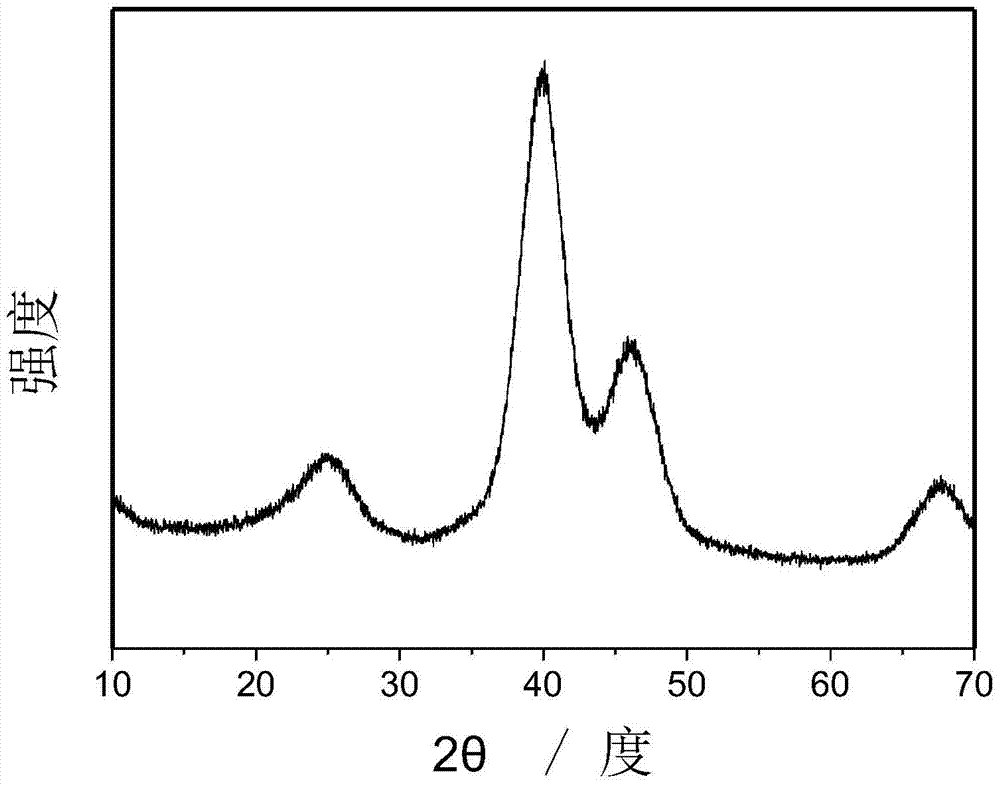
[0063] Mix 5.8 mg of nano-platinum-supported activated carbon material, 3 mL of acetonitrile, and 1.2 mmol of hydrogen peroxide, and stir at 25°C for 30 minutes under ultraviolet light, and for 5 hours without ultraviolet light; the product is centrifuged and dried to obtain surface cyanide. modified Pt / C catalysts. Figure 3(a) is the XRD spectrum of the Pt / C catalyst modified by surface cyano groups. There are no other peaks in the figure, and it can be seen that the modification of cyano groups is only on the metal surface. Mix 5.8 mg nano-gold-loaded activated carbon material, 3 mL acetonitrile, and 1.2 mmol hydrogen peroxide, and stir for 4 hours at 25 °C under ultraviolet light; the product is centrifuged and dried to obtain a modified Au / C catalyst. Figure 3(b) is the XRD spectrum of the modified Au / C catalyst. There are other peaks in the figure, which shows that other substances are gradually formed. If the reaction time is increased, it will eventually become other su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com