Preparation method of foot-and-mouth disease vaccines
A foot-and-mouth disease vaccine and antigen technology, which is applied in the preparation of foot-and-mouth disease vaccine and the purification process of foot-and-mouth disease vaccine cell culture liquid, can solve the problems of increasing the risk of cross-contamination, large antigen loss, and increasing impurity load.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] ——Preparation of foot-and-mouth disease type O and Asia1 bivalent inactivated vaccines
[0042] Will BHK 21 Suspension cells were respectively inoculated with the cell culture medium of foot-and-mouth disease virus ONXC / 92 strain and Asia1 / JSL / GSZY / 06 strain, concentrated and purified by the following method, and the inactivated two virus antigen solutions were mixed and diluted, and then added 206 The adjuvant is mixed and emulsified to make a vaccine for the prevention of bovine O-type and Asia1-type foot-and-mouth disease. BHK 21 Cells were purchased from China Veterinary Drug Administration.
[0043] 1. Provide foot-and-mouth disease vaccine cell culture fluid and depth filter device.
[0044] (1) The foot-and-mouth disease virus used for vaccine production of the present invention is ONXC / 92 strain and Asia1 / JSL / GSZY / 06 strain, purchased from Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. The two strains were respectively inocu...
Embodiment 2
[0055] ——Preparation of Foot-and-Mouth Disease Type O Bivalent Inactivated Vaccine
[0056] BHK21 suspension cells were inoculated with O / Mya98 / XJ / 2010 and O / GX / 09-7 strain cell culture fluid respectively, and the two kinds of virus antigen solutions after concentration, purification and inactivation were mixed and diluted by the following method, and then Add 206 adjuvant to mix and emulsify to make a vaccine, which is used for the prevention of porcine O-type foot-and-mouth disease.
[0057] 1. Provide foot-and-mouth disease vaccine cell culture fluid and depth filter device.
[0058] (1) The virus used in this vaccine is foot-and-mouth disease virus O / Mya98 / XJ / 2010 strain and O / GX / 09-7 strain, purchased from Lanzhou Veterinary Research Institute. The two strains were respectively inoculated with BHK21 cell suspension culture to obtain the antigen solution.
[0059] (2) The device used for deep filtration uses diatomite as a filter aid, and the feed liquid is in contact wi...
Embodiment 3
[0070] ——Quality inspection of foot-and-mouth disease ONXC / 92 strain and Asia1 / JSL / GSZY / 06 bivalent inactivated vaccine
[0071] The test is divided into two groups, the group A adopts the original purification method to prepare the vaccine, and the group B adopts the technology used in the present invention to prepare the vaccine.
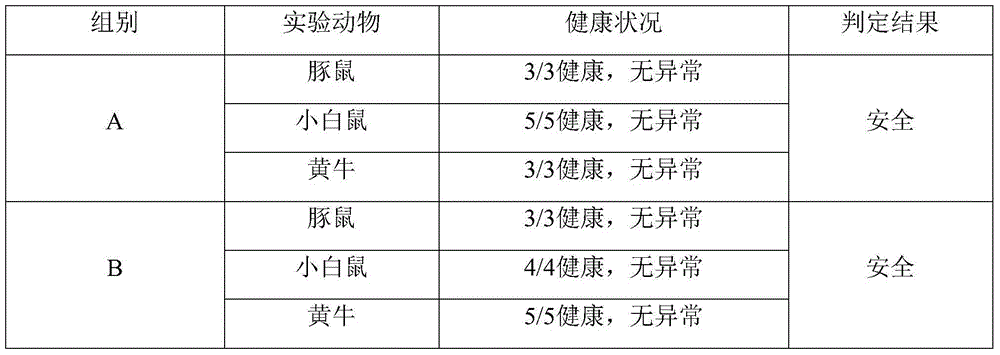
[0072] 1. The mensuration of effective antigen content of vaccine: detect foot-and-mouth disease 146S antigen content with sucrose density gradient method, get 10 samples respectively before and after each group of purification, get average value, compare two experimental groups 146S antigen content, as shown in table 1, result It shows that the loss of the antigen purified by the present invention is small, and the content of the effective antigen obtained is high.
[0073] Table 1 Comparison of viral antigen content in vaccines prepared by two processes
[0074] group
146S before purification (μg / ml)
Purified 146S (μg / ml)
e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com