A kind of biofilm for treating glaucoma and preparation method thereof
A glaucoma treatment and biofilm technology, applied in medical science, prosthesis, etc., can solve the problems of fragility, inability to guarantee biological activity, and reduction of active components of amniotic membrane, so as to ensure biological safety, good clinical application prospects, and inhibit scarring. effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A method for preparing a biofilm for glaucoma treatment, the steps of which are as follows:
[0049] (1)Quick collection of materials
[0050] The amniotic membrane rapid extraction device is used to quickly remove the amniotic membrane attached to the placenta around the umbilical cord within 1 minute, and quickly refrigerate at 2°C, and transport it to the clean workshop for subsequent processing within 1 hour;
[0051] (2) Stamping and cutting
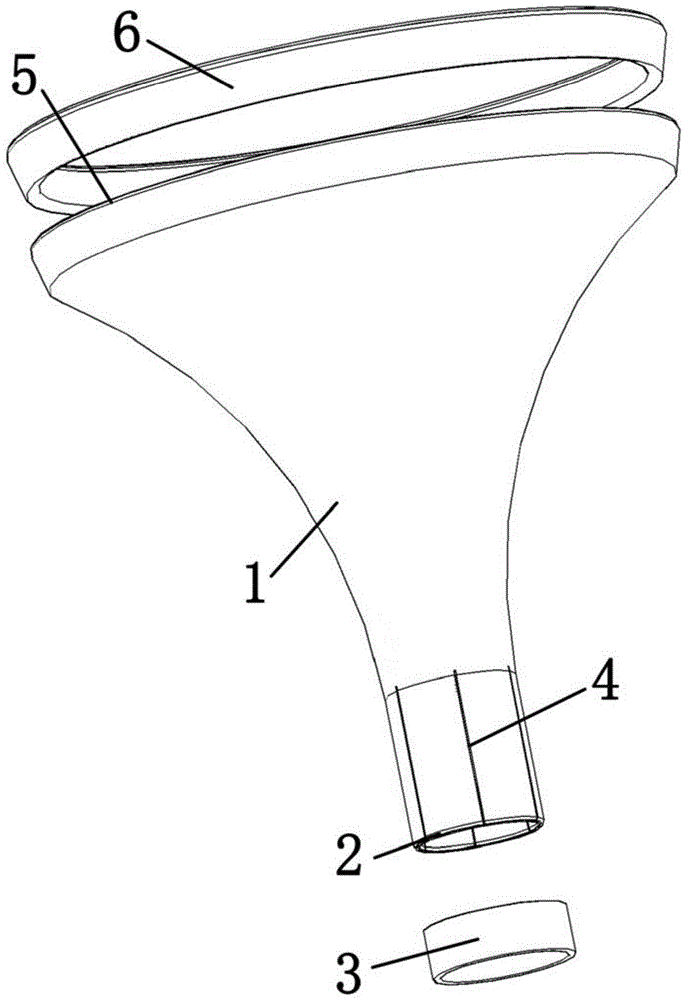
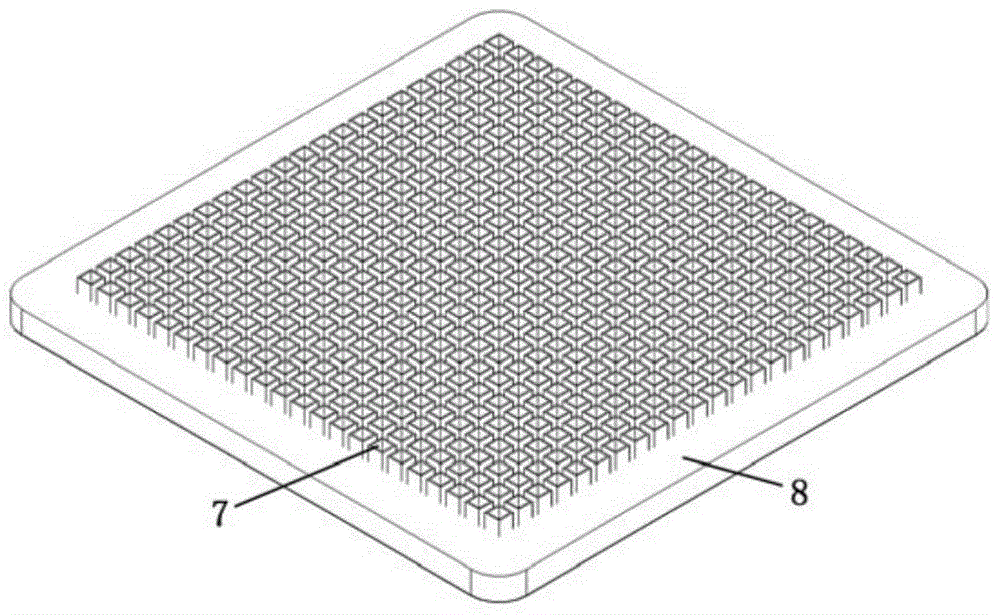
[0052] The amnion epithelium obtained in step (1) was quickly spread on the nitrocellulose filter membrane, and the amnion material obtained in step 1 was cut into a square amnion with a length of 3 mm and a width of 3 mm within 1 minute using a punching amnion cutting device Diaphragm;
[0053] (3) Compound thickening
[0054] Soak the amniotic membrane sheet prepared in step (2) in an aqueous solution of hyaluronic acid with a molecular weight of 1 million pharmaceutical grades at a concentration of 1% by mass for 1 minu...
Embodiment 2
[0059] A method for preparing a biofilm for glaucoma treatment, the steps of which are as follows:
[0060] (1)Quick collection of materials
[0061] The amniotic membrane rapid extraction device is used to quickly remove the amniotic membrane attached to the placenta around the umbilical cord within 3 minutes, and quickly refrigerate at 8°C, and transport it to the clean workshop for subsequent processing within 1 hour.
[0062] (2) Stamping and cutting
[0063] Spread the amnion epithelium obtained in step (1) upwards on the nitrocellulose filter quickly, and cut the amnion material obtained in step 1 into a rectangle with a length of 10 mm and a width of 3 mm within 3 minutes using a punching amnion cutting device Amniotic film.
[0064] (3) Compound thickening
[0065] Soak the amniotic membrane sheet prepared in step (2) in a pharmaceutical-grade high-viscosity sodium carboxymethylcellulose aqueous solution with a mass percent concentration of 5% for 3 minutes to thick...
Embodiment 3
[0070] A method for preparing a biofilm for glaucoma treatment, the steps of which are as follows:
[0071] (1)Quick collection of materials
[0072] The amniotic membrane rapid extraction device is used to quickly remove the amniotic membrane attached to the placenta around the umbilical cord within 2 minutes, and quickly refrigerate at 4°C, and transport it to the clean workshop for subsequent processing within 1 hour.
[0073] (2) Stamping and cutting
[0074] The amnion epithelium obtained in step (1) was quickly spread on the nitrocellulose filter membrane, and the amniotic membrane material obtained in step 1 was cut into circular amnion membrane pieces with a diameter of 6 mm within 2 minutes using a punching amnion membrane cutting device .
[0075](3) Compound thickening
[0076] Soak the amniotic membrane sheet prepared in step (2) in a 3% pharmaceutical-grade hyaluronic acid aqueous solution with a molecular weight of 1.5 million for 2 minutes to thicken the amni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com