Synthetic method for romatic carbamic acid ester
A technology for synthesizing carbamic acid and carbamic acid, which is applied to the preparation of carbamic acid derivatives, organic chemical methods, chemical instruments and methods, etc., can solve the problems of not being easy to obtain phenol and the small range of substrates, and achieve non-toxic raw materials and methods Simple, safe and simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
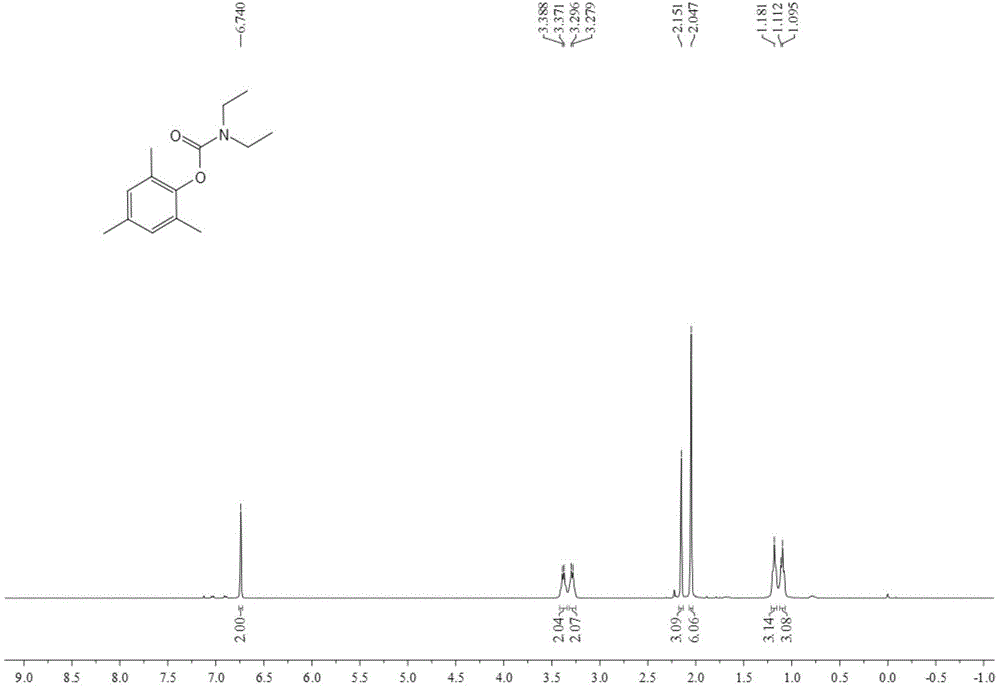
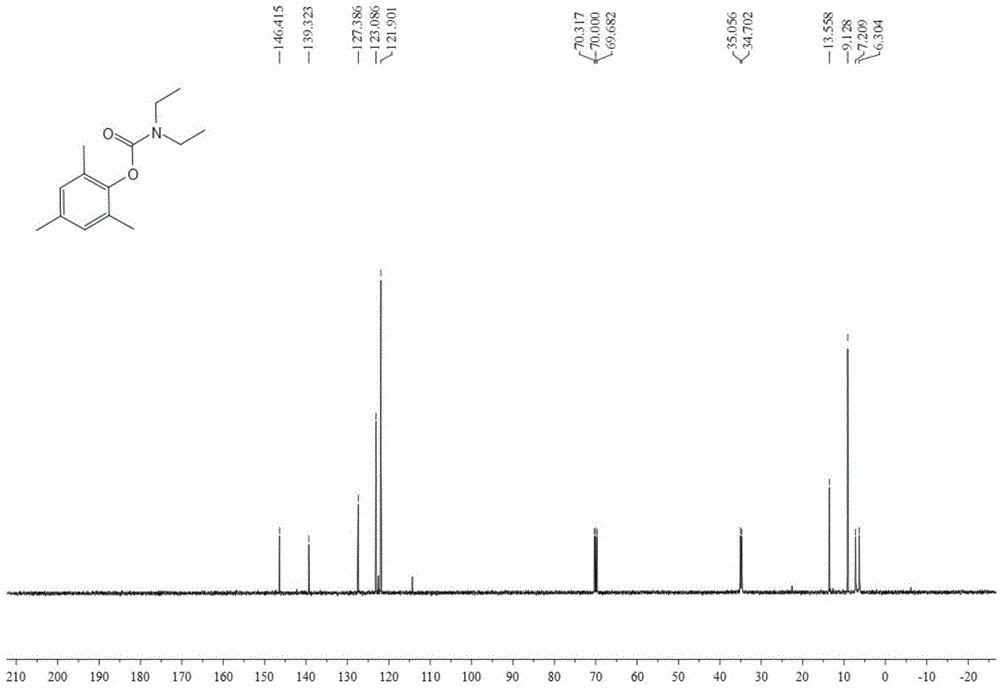
[0045]Add 0.25 mmol of bis(2,4,6-trimethylphenyl)iodonium trifluoromethanesulfonate, 2.5 mmol of diethylamine, 0.5 mmol of 1,8-diazabis Cyclo[5.4.0]undec-7-ene (DBU), 3 mL DMF, filled with 4 MPa of CO 2 , after stirring and reacting at 80°C for 12 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2 . The reaction solution was extracted with ethyl acetate, the solvent was removed by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 30:1 , yield 72%.
Embodiment 2
[0047] Add 0.25 mmol of bis(2,4,6-trimethylphenyl)iodonium trifluoromethanesulfonate, 2.5 mmol of diethylamine, 0.5 mmol of 1,4-diazabis Cyclo[2.2.2]octane (DABCO), 3 mL of acetonitrile, filled with 4 MPa of CO 2 , after stirring and reacting at 80°C for 12 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2 . The reaction solution was extracted with ethyl acetate, the solvent was removed by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 30:1 , yield 63%.
Embodiment 3
[0049] Add 0.25 mmol of bis(2,4,6-trimethylphenyl)iodonium trifluoromethanesulfonate, 2.5 mmol of diethylamine, 0.5 mmol of 1,8-diazabis Cyclo[5.4.0]undec-7-ene (DBU), 3 mL of acetonitrile, filled with 0.7 MPa of CO 2 , after stirring and reacting at 40°C for 12 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2 . The reaction solution was extracted with ethyl acetate, the solvent was removed by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 30:1 , yield 41%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com