Axial ALA-modified silicon phthalocyanine and preparation method and application thereof
A silicon phthalocyanine, axial technology, applied in the field of axial ALA-modified silicon phthalocyanine and its preparation, can solve the problems of high skin phototoxicity, clinical application limitations, unstable composition, etc., and achieve clear structure and tissue penetration Enhanced capacity and large molar absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
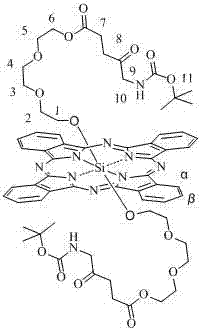
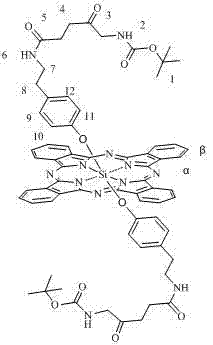
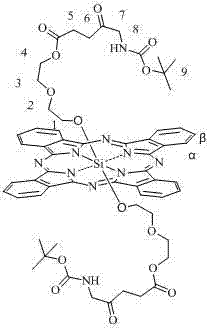
[0029] The method for preparing axial ALA-modified silicon phthalocyanine of the present invention is characterized in that: (1) bis[2-(2-hydroxyethoxy)ethoxy] silicon phthalocyanine or bis[2-(2-( 2-Hydroxyethoxy)ethoxy)ethoxy]silicon phthalocyanine or bis[4-(2-aminoethyl)phenoxy]silicon phthalocyanine and N-Boc-5-aminolevulinic acid are reacted The molar ratio of the two materials is 1:2~4 (preferably 1:3). (2) Toluene, xylene or dioxane is used as solvent, and the amount of solvent is 1mmol of phthalocyanine silicon. 50~150ml of solvent (preferably 90ml) is required. Under the protection of nitrogen, react at 20~30℃ for 24~48 hours. Solvent cleaning and column chromatography are used to separate and remove excess raw materials and impurities to obtain corresponding silicon phthalocyanine modified with Boc-ALA and oligoethylene glycol or aminoethylphenoxy.
[0030] The silicon phthalocyanine provided by the present invention can be used to prepare photodynamic drugs or photosen...
Embodiment 1
[0037] Synthesis and physicochemical properties of bis[2-(2-hydroxyethoxy)ethoxy] silicon phthalocyanine (structure shown in the following formula):
[0038]
[0039] Under the protection of nitrogen, weigh out 90 mg (147.17 μmol) of dichlorosilicon phthalocyanine, 1 ml of diethylene glycol, and NaH added to toluene or xylene or 20-50 ml of dioxane (preferably toluene, 30 ml), Reflux for 12-24 hours (preferably 18 hours). The solvent was removed by vacuum rotary evaporation. Dissolve with a small amount of DMF and filter. The filter cake is unreacted dichlorosilica phthalocyanine. A large amount of water is added to the filtrate to precipitate out. The filter cake is vacuum-dried with an organic membrane. Then re-dissolve with dichloromethane and centrifuge to obtain the supernatant of the crude product. It was further subjected to silica gel column chromatography, using ethyl acetate as the eluent, and the target components were collected, evaporated to dryness and vacuum drie...
Embodiment 2
[0042] Synthesis and physicochemical properties of an axial ALA modified silicon phthalocyanine (structure shown in the following formula):
[0043]
[0044] Under the protection of nitrogen, bis[2-(2-hydroxyethoxy)ethoxy]silicon phthalocyanine 50mg (66.7μmol), N-Boc-5-aminolevulinic acid 46.3mg (200.1μmol), DMAP 24.4 mg (200.1 μmol), HOBt 27.0 mg (200.1 μmol), EDCI 38.4 mg (200.1 μmol), 100 μl triethylamine, disperse in 6 ml of dichloromethane and stir for 24 to 48 hours (preferably 36 hours). After the reaction is over, add appropriate amount of water for extraction three times, remove water with anhydrous sodium sulfate, evaporate the solvent, and dry in vacuum. Dissolve in a small amount of dichloromethane and pass through a silica gel column. The eluent is dichloromethane: ethyl acetate=1:1. Collect the target components and evaporate to dryness. Re-dissolve it with a small amount of tetrahydrofuran, then collect the target component through an X3 gel column and evaporate i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com