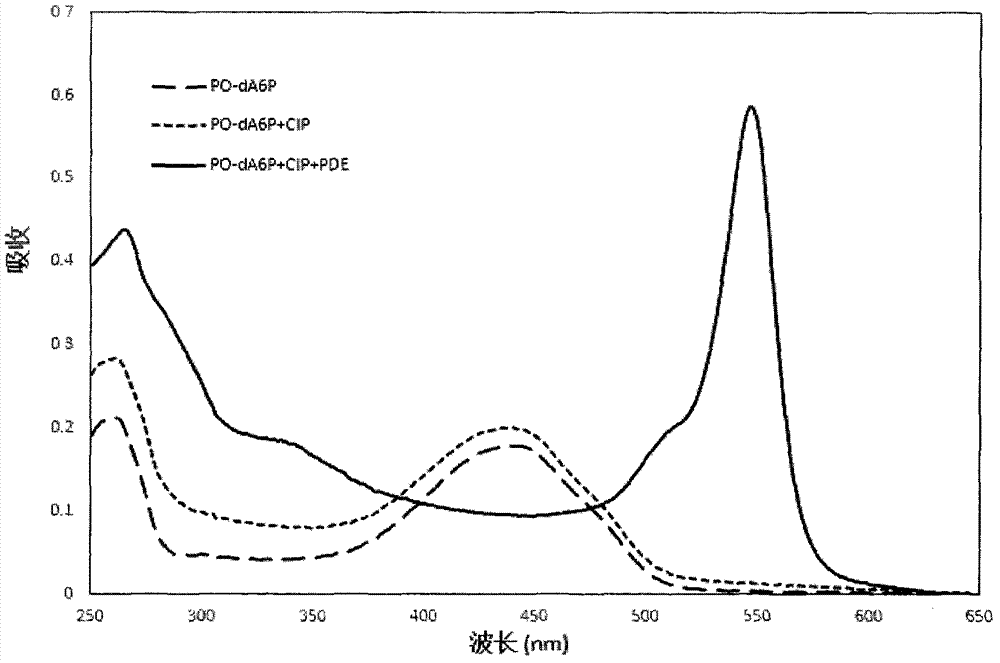
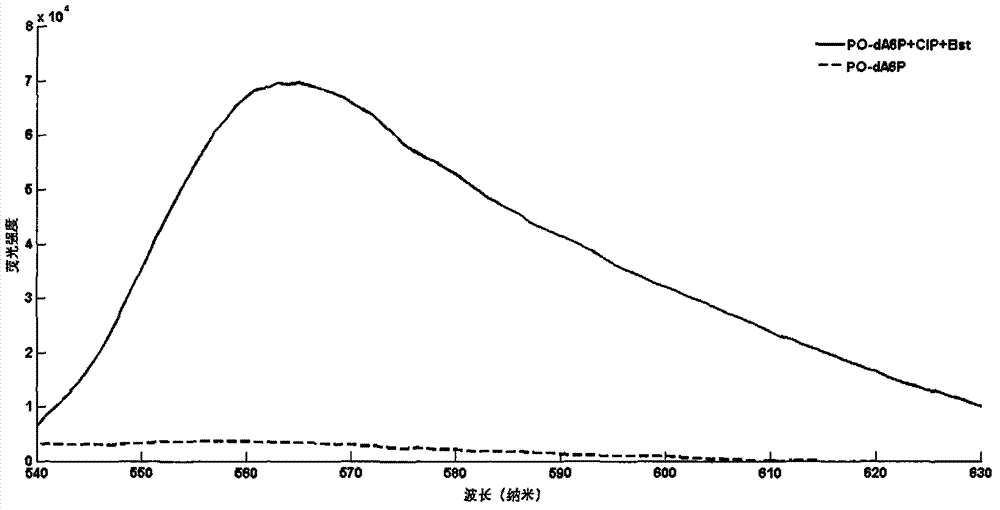
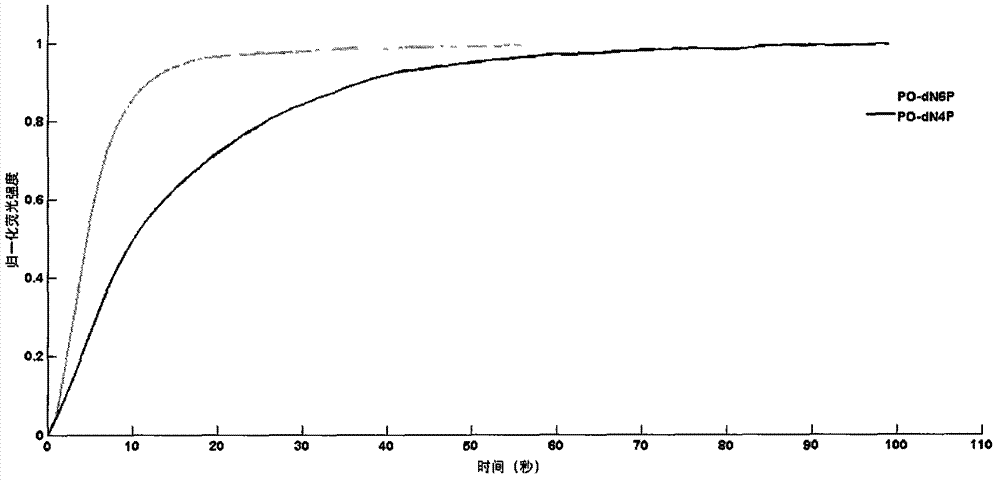
Novel polymerase substrate, fluorescence generable polyphosphoric acid end-labeled nucleotide, and its application
A fluorescent labeling and nucleotide technology, which is applied in the direction of sugar derivatives, luminescent materials, sugar derivatives, etc., can solve the problems of difficult synthesis of long phosphate chains and indirect synthesis methods, so as to improve product yield and avoid by-products , the effect of polyphosphate chain length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Provided is a fluorescein analogue-carbon bridge-substituted fluorescein or anthracene-based fluorescein, which has the characteristics of long wavelength, fluorescence generation, simple and easy derivatization, high extinction coefficient and quantum yield, and has the advantages of Following structural formula (1):
[0041]
[0042] A fluorescent dye based on anthracene structure is characterized in that: it has a structure as shown in general formula (1), wherein R 0 may be selected from -H, phosphate, substituted phosphate; R 1 , R 5 Can be independently selected from -H, fluorine, chlorine, aryl, substituted aryl, C1-C6 alkyl, substituted C1-C6 alkyl, C1-C6 alkoxy, substituted C1-C6 alkoxy; R 2-4 , R 6-8 , R 11-13 Can be independently selected from -H, fluorine, chlorine, bromine, aryl, substituted aryl, heteroaryl, -CO 2 H, -CO 2 R, -SO 3 H, -SO 3 R, -CH 2 CO 2 H, -CH 2 CO 2 R, -CH 2 SO 3 H, -CH 2 SO 3 R, -CH 2 NH 2 、-CH 2 NHR, -NO 2 , C1-C...
Embodiment 2
[0052] According to the compound described in embodiment 1, it can specifically be:
[0053]
Embodiment 3
[0055] According to the compound described in embodiment 2, its synthetic method can specifically be as follows:
[0056]
[0057] Reagents and reaction conditions: i) THF, -40°C; ii) DCM, PCC, diatomaceous earth; iii) ZnMe 2 , TiCl 4 , DCM-40°C; iv) t-Buli, THF; o-tolualdehyde; v) DCM, PCC; vi) BBr 3 ;MeSO 3 h
[0058] 1). Synthesis of (2-bromo-5-methoxyphenyl)-3-methoxybenzyl alcohol 1a
[0059]
[0060] In a 250ml dry round bottom bottle equipped with a constant pressure dropping funnel, cool the 80ml dry tetrahydrofuran solution of m-methoxyphenylmagnesium chloride to -40°C, and dissolve 3-methoxy-o-bromobenzaldehyde under the protection of argon. (10.8g) of 20ml dry tetrahydrofuran solution was added dropwise into the reaction flask through a constant pressure dropping funnel, kept at -40°C and stirred for 2-6 hours, and the reaction was monitored by TLC. After the disappearance of the raw material 3-methoxy-o-bromobenzaldehyde The reaction was stopped, and 20 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com