The ubiquitin-binding domain of a secreted protein from Mycobacterium tuberculosis
A technology of Mycobacterium tuberculosis and binding domain, applied in the field of cell biology, can solve the problem that the influence of PtpA protein on cell signaling pathway is not clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 The Ubiquitin Binding Domain (UIML Domain) of Mycobacterium Tuberculosis Secretory Protein PtpA
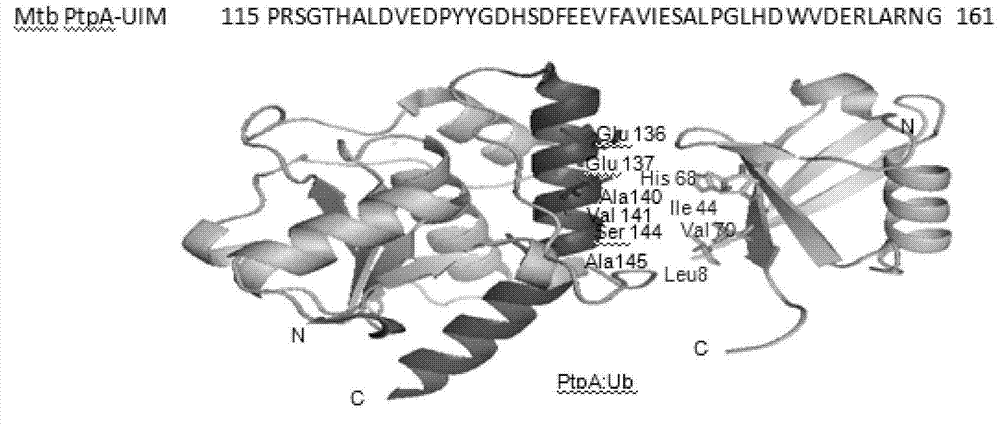
[0022] The domain of the interaction between Mycobacterium tuberculosis secreted protein PtpA and ubiquitin, its amino acid sequence is: PRSGTHALDVEDPYYGDHSDFEEVFAVIESALPGLHDWVDERLARNG. The UIML domain and its structural analogues can be used in the screening of anti-tuberculosis drugs.
[0023] The preparation method of the PtpAUIML domain protein is to construct the recombinant plasmid pGEX-6P-1-PtpA-UIML containing the PtpAUIML domain gene, and transform the plasmid into Escherichia coli BL21, and use IPTG to induce protein expression at 30°C; Collect the supernatant after high-speed centrifugation; slowly flow the supernatant through the filled Glutathione-Sepharose beads (GE), then add column washing buffer to fully wash the column, and finally add 2-3ml eluent to elute Protein; add the collected eluate into a 10KD protein concentrator tube (millipore), cent...
Embodiment 2
[0024] Example 2 Mycobacterium tuberculosis secretory protein PtpAUIML domain directly interacts with ubiquitin
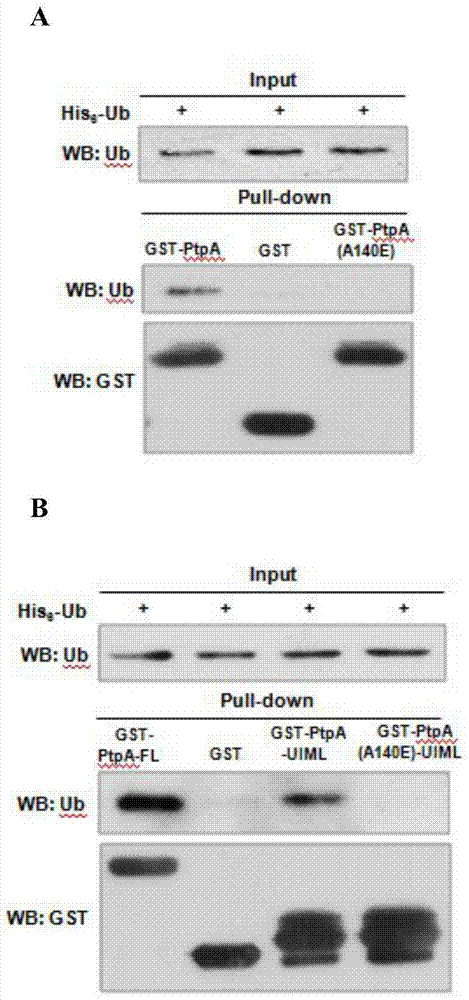
[0025] Through the crystal structure analysis of the known PtpA and Ub proteins, it was found that there was an amino acid sequence (UIML domain) similar to the eukaryotic UIM domain in the PtpA protein ( figure 1 ), because there is a hydrophobic amino acid sequence similar to the eukaryotic UIM domain in this sequence, we think that this domain may be related to the interaction of ubiquitin, and it is the first prokaryotic UIM domain we discovered. The UIML domain is not consistent with the eukaryotic UIM domain that has been reported so far, and it is the first time we have discovered it in a prokaryotic protein. At the same time, through crystal structure analysis, we think that the A140 site may be the key site of the hydrophobic interaction between PtpA protein and ubiquitin.
[0026] Through in vitro experiments, the PtpA (A140E) protein with A140 site muta...
Embodiment 3
[0029]Example 3 Ubiquitin loses the ability to regulate the enzyme activity of the phosphatase PtpA (A140E) mutant
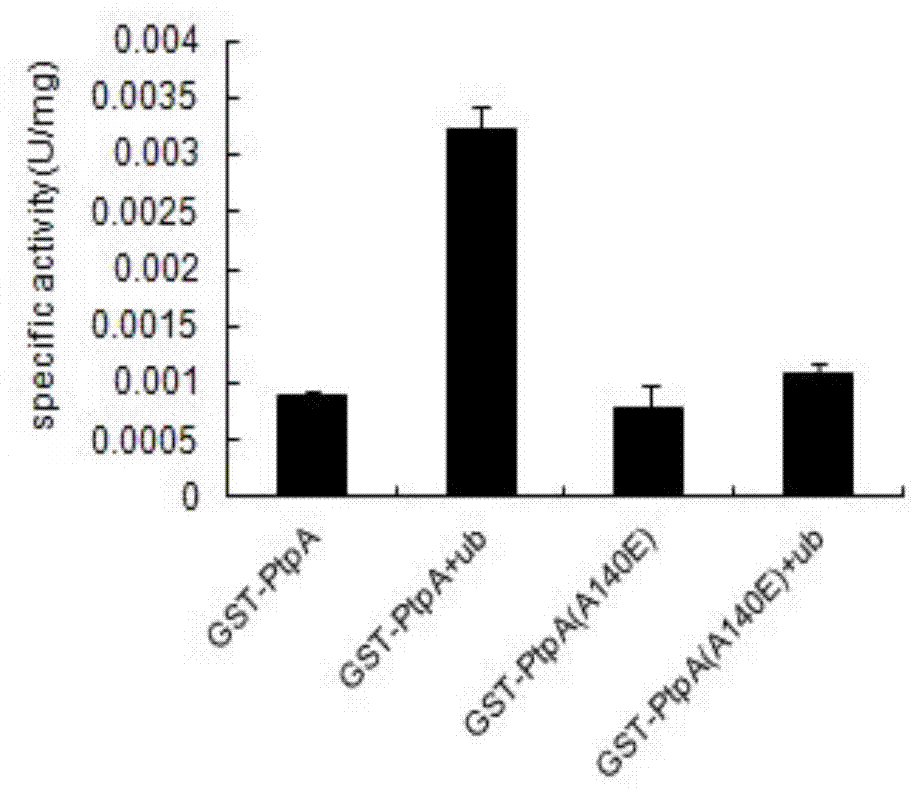
[0030] In the Chinese invention patent application 201310466907.2, it is recorded that Ub has a strong activation effect on the phosphatase activity of PtpA, thereby enhancing its activity to dephosphorylate the substrates p-JNK and p-P38.
[0031] After performing the same enzyme activity experiment, we found that because PtpA (A140E) lost the ability to interact with ubiquitin, its phosphatase activity was no longer regulated by ubiquitin ( image 3 ). Also in the dephosphorylation reaction in vitro, we also obtained the same result, that is, the dephosphorylation activity of PtpA (A140E) on the substrates p-JNK and p-P38 was no longer regulated by ubiquitin ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com