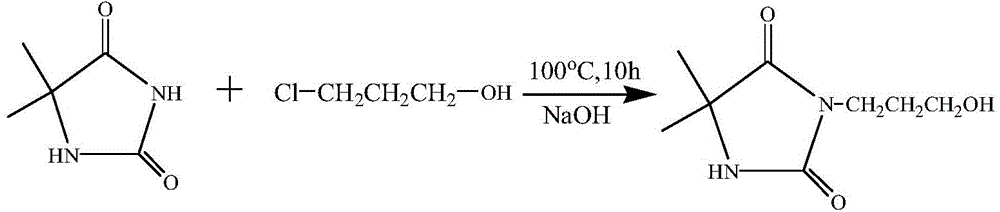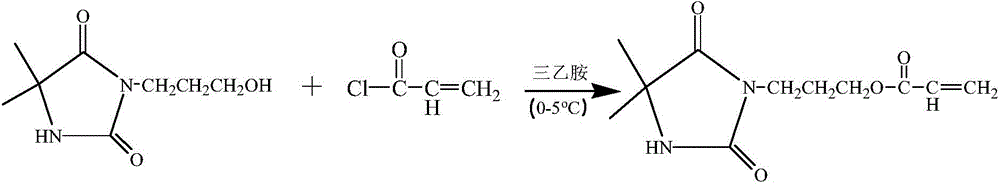Halamine double bond hydantoin antiseptic, and preparation method and application thereof
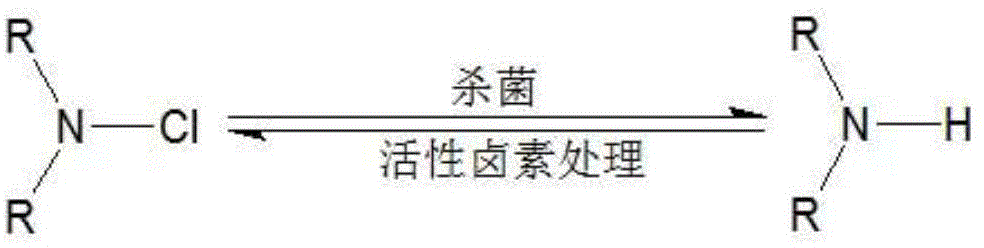
A technology of antibacterial agent and halamine, which is applied in the production field of functional textiles, can solve problems such as harsh process conditions, complicated processing process, and increased raw material cost, and achieve excellent antibacterial performance, simplified process, and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] React with 0.1 mol of NaOH and 0.1 mol of 5,5-dimethylhydantoin as raw materials, then add deionized water, and stir for 15 minutes to obtain a colorless and transparent solution, which is the sodium salt of 5,5-dimethylhydantoin. Then add 0.1mol of 3-chloro-1-propanol, raise the temperature to 100°C and add a magnetic stirrer to stir the solution, and react for 10h under the condition of condensation and reflux to obtain 3-(3-hydroxypropyl)-5,5 - crude product of dimethylhydantoin. The deionized water was distilled off under reduced pressure using a rotary evaporator to obtain a solid mixture. Dissolve in acetone, filter, and spin evaporate the filtrate to obtain a solid that is 3-(3-hydroxypropyl)-5,5-dimethylhydantoin. Put the 3-(3-hydroxypropyl)-5,5-dimethylhydantoin obtained in the first step into 100ml of tetrahydrofuran, and use 0.1mol of triethylamine as an acid scavenger. Under the condition of ice bath, 0.1 mol of acryloyl chloride was added dropwise and all...
Embodiment 2
[0047] React with 0.1 mol of NaOH and 0.1 mol of 5,5-dimethylhydantoin as raw materials, then add deionized water, and stir for 15 minutes to obtain a colorless and transparent solution, which is the sodium salt of 5,5-dimethylhydantoin. Then add 0.1mol of 3-chloro-1-propanol, raise the temperature to 100°C and add a magnetic stirrer to stir the solution, and react for 12h under the condition of condensation and reflux to obtain 3-(3-hydroxypropyl)-5,5 - crude product of dimethylhydantoin. The deionized water was distilled off under reduced pressure using a rotary evaporator to obtain a solid mixture. Dissolve in acetone, filter, and spin evaporate the filtrate to obtain a solid that is 3-(3-hydroxypropyl)-5,5-dimethylhydantoin. Put the 3-(3-hydroxypropyl)-5,5-dimethylhydantoin obtained in the first step into 100ml of tetrahydrofuran, and use 0.1mol of triethylamine as an acid scavenger. Under the condition of ice bath, 0.1 mol of acryloyl chloride was added dropwise and all...
Embodiment 3
[0049] React with 0.1 mol of NaOH and 0.1 mol of 5,5-dimethylhydantoin as raw materials, then add deionized water, and stir for 15 minutes to obtain a colorless and transparent solution, which is the sodium salt of 5,5-dimethylhydantoin. Then add 0.1mol of 3-chloro-1-propanol, raise the temperature and add a magnetic stirrer to stir the solution, and react for 15 hours at 95°C to obtain 3-(3-hydroxypropyl)-5,5-dimethyl Crude product of Ghenyne. The deionized water was distilled off under reduced pressure using a rotary evaporator to obtain a solid mixture. Dissolve in acetone, filter, and spin evaporate the filtrate to obtain a solid that is 3-(3-hydroxypropyl)-5,5-dimethylhydantoin. Put the 3-(3-hydroxypropyl)-5,5-dimethylhydantoin obtained in the first step into 100ml of tetrahydrofuran, and use 0.1mol of triethylamine as an acid scavenger. Under the condition of ice bath, 0.1 mol of acryloyl chloride was added dropwise and allowed to react for 1 h under the condition of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com