Synthetic method of 5-chloro-1-indanone
A technology of indanone and chlorophenylpropionic acid, which is applied in the field of synthesis of 5-chloro-1-indanone, can solve the problems of harsh reaction conditions, expensive catalysts, environmental pollution, etc., and achieve simple treatment, easy process, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
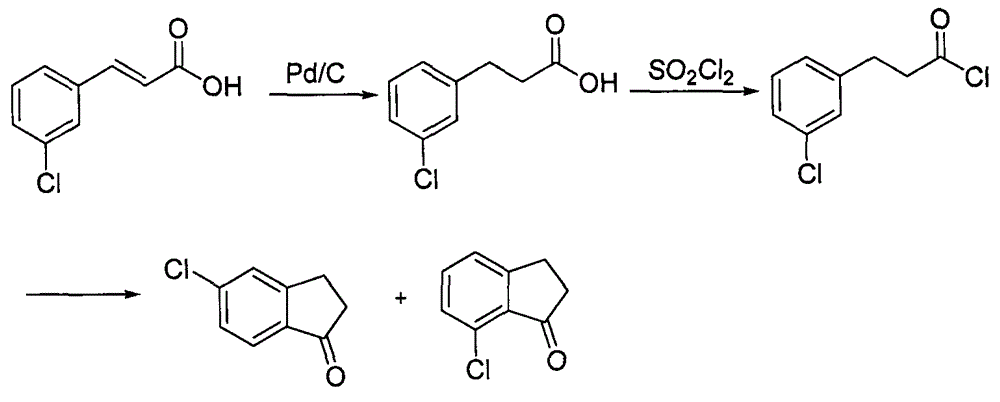
[0029] (1) The preparation of m-chlorophenylpropionic acid has the following technical process:
[0030]
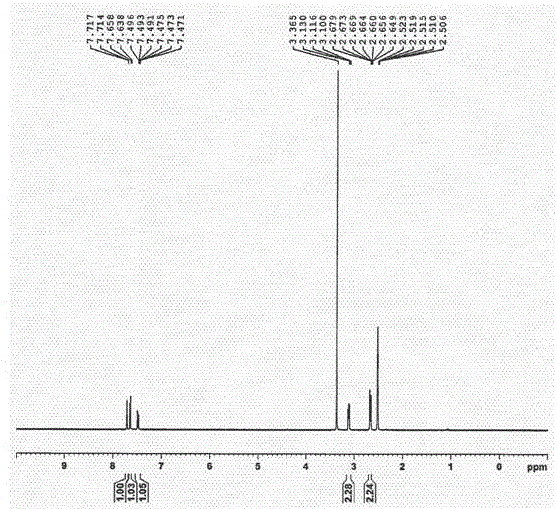
[0031] In a 100mL three-necked flask, add 40g of formic acid, 29g of diethylamine, m-chlorobenzaldehyde (10g, 0.071mol) and malonic acid (8.0g, 0.077mol), stir to dissolve, then heat up to 150°C, and reflux the reaction , after the reaction was complete as detected by TLC, pour it into 400mL of ice water and stir, then adjust the pH to 3-4 with concentrated hydrochloric acid, filter, and recrystallize the filter cake with ethyl acetate to obtain 10.4g of the target product, with a yield of 79.6% and a melting point of 71.5- 75.2°C.
[0032] (2) The preparation of 5-chloro-1-indanone has the following technical process:
[0033]
[0034] In a 100mL flask, add m-chlorophenylpropionic acid (9g, 0.049mol), 40mL of dichloromethane, malonyl chloride (5.6mL, 0.058mol), stir for 10min, then slowly add 9.4g of zinc chloride, react for about 2h, TLC After the completion of ...
Embodiment 2
[0036] (1) The preparation of m-chlorophenylpropionic acid has the following technical process:
[0037]
[0038] In a 100mL three-necked flask, add 40g of formic acid, 29g of diethylamine, m-chlorobenzaldehyde (10g, 0.071mol) and malonic acid (8.9g, 0.085mol), stir to dissolve, then heat up to 150°C, and reflux the reaction , after the reaction was complete as detected by TLC, pour it into 400mL of ice water and stir, then adjust the pH to 3-4 with concentrated hydrochloric acid, filter, and recrystallize the filter cake with ethyl acetate to obtain 10.8g of the target product, with a yield of 82.3% and a melting point of 71.9- 75.8°C.
[0039] (2) The preparation of 5-chloro-1-indanone has the following technical process:
[0040]
[0041] In a 100mL flask, add m-chlorophenylpropionic acid (9g, 0.049mol), 40mL of dichloromethane, malonyl chloride (5.8mL, 0.059mol), stir for 10min, then slowly add 9.7g of zinc chloride, react for about 2h, TLC After the completion of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com