Phenyl quinoline derivative iridium (III) complex based on piperidine or morpholine methylene substitution, and preparation method and application thereof
A technology of morpholine methylene and phenylquinoline iridium, applied in the field of preparation of phenylquinoline iridium complexes, to achieve the effects of reducing signal-to-noise ratio, excellent pH value response properties, and improving detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
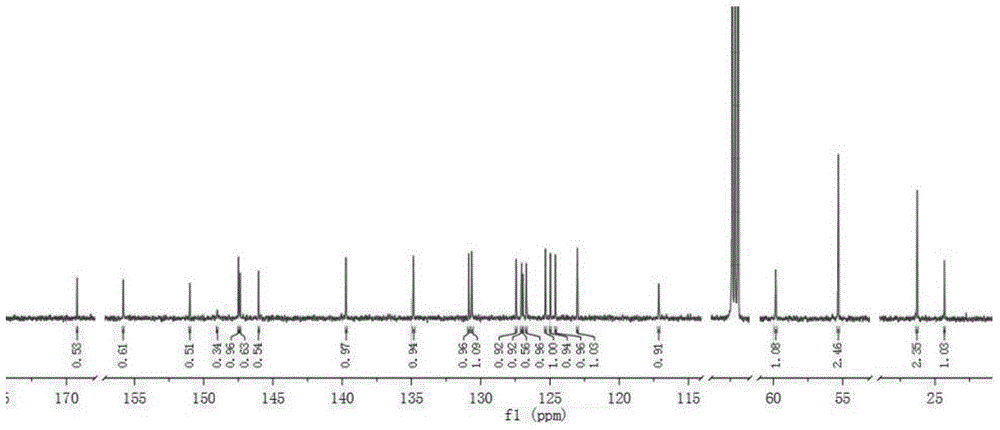
[0036] Embodiment 1: Complex [Ir(PQ-C-N) 2 (bpy)] + PF 6 - Synthesis
[0037]
[0038] (1) Synthesis of 2-bromo-4-bromomethylquinoline
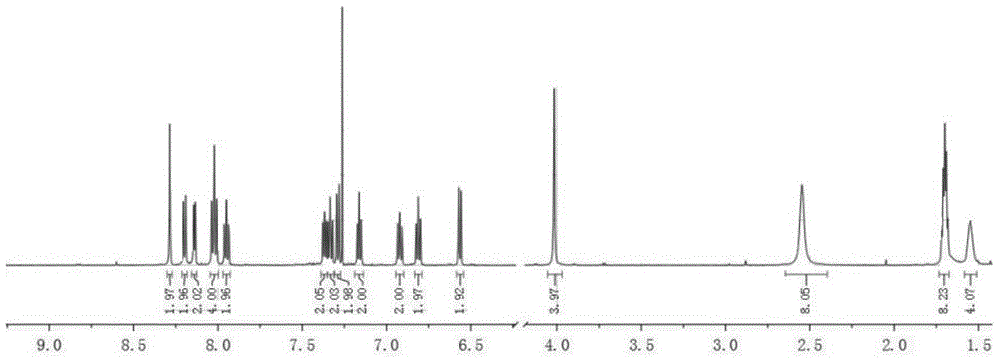
[0039] Take 1.11g of 2-bromo-4-methylquinoline, 0.98g of N-bromosuccinimide (NBS), 240mg of benzoyl peroxide (BPO), and 50mL of carbon tetrachloride, and add them to the belt stirring In a three-necked flask with nitrogen gas, reflux at 80°C for 24h, spin dry under reduced pressure, and use petroleum ether / ethyl acetate (V:V=100:1) column chromatography to obtain a colorless crystalline powder with a yield of 49 %. 1 H NMR (600MHz, DMSO-d 6 )δ(ppm): 8.24(d; J=8.40Hz; 1H); 8.01(d; J=8.40Hz; 1H); 7.89(s; 1H); 7.84(t; J=7.20Hz; 1H); 7.76 (t; J = 7.20 Hz; 1H); 5.15 (s; 2H).
[0040] (2) Synthesis of 2-bromo-4-(piperidin-1-ylmethyl)quinoline
[0041] Take 3.01g of 2-bromo-4-bromomethylquinoline, 0.90g of piperidine, 5g of anhydrous potassium carbonate, and 100mL of anhydrous acetonitrile, and add them in turn to a three-necked flask wi...
Embodiment 2
[0051] Embodiment 2: Complex [Ir(PQ-C-NO) 2 (bpy)] + PF 6 - Synthesis
[0052]
[0053] (1) Synthesis of 2-bromo-4-(morpholin-1-ylmethyl)quinoline
[0054] Take 3.01g of 2-bromo-4-bromomethylquinoline, 0.91g of morpholine, 5g of anhydrous potassium carbonate, and 100mL of anhydrous acetonitrile, and add them in turn to a three-necked flask with a stirring bar, react at 25°C for 24h, and then depressurize Spin to dry, and use petroleum ether / ethyl acetate (V:V=10:1) column chromatography to obtain a colorless crystalline powder with a yield of 90%.
[0055] (2) Synthesis of 2-phenyl-4-(morpholin-1-ylmethyl)quinoline
[0056] Take 2-bromo-4-(morpholin-1-ylmethyl)quinoline 1.29g, phenylboronic acid 0.52g, Pd(PPh 3 ) 4 160mg, 10mL saturated aqueous sodium carbonate solution, 10mL absolute ethanol, and 30mL toluene were added to a three-necked flask with a stirring bar in turn, nitrogen was passed, and after reflux reaction for 14 hours, after washing and drying, spin-dry...
Embodiment 3
[0064] Embodiment 3: Complex [Ir(4FPQ-C-N) 2 (bpy)] + PF 6 - Synthesis
[0065]
[0066] (1) Synthesis of 2-(4-fluorophenyl)-4-(piperidin-1-ylmethyl)quinoline
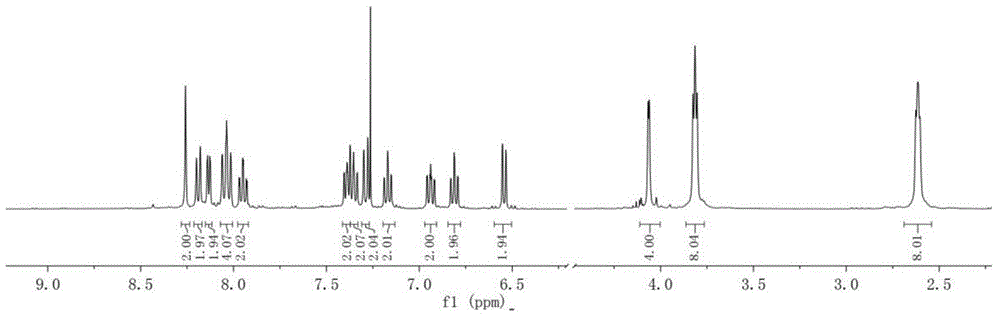
[0067] Get 2-bromo-4-(piperidin-1-ylmethyl) quinoline 1.29g synthesized in Example 1, 591mg of 4-fluorophenylboronic acid, Pd(PPh 3 ) 4 160mg, 10mL saturated aqueous sodium carbonate solution, 10mL absolute ethanol, and 30mL toluene were added to a three-necked flask with a stirring bar in turn, nitrogen was passed, and after reflux reaction for 14 hours, after washing and drying, spin-drying under reduced pressure, and using petroleum ether / ethyl acetate (V:V=10:1) Column chromatography yielded a white crystalline powder with a yield of 91%. 1 H NMR (400MHz, CDCl 3 )δ(ppm): 8.23(dd; J=0.80Hz; J=8.40Hz; 1H); 8.21-8.16(m; 2H); 8.16(dd; J=0.40Hz; J=8.40Hz; 1H); 7.90 (t; J=0.40Hz; 1H); 7.71(ddd; J=1.60Hz; J=6.80Hz; J=8.40Hz; 1H); 7.53(ddd; J=1.20Hz; J=6.80Hz; J=8.40 Hz; 1H); 7.24-7.18(m; 2H); 3.93(d; J=0.40Hz;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com