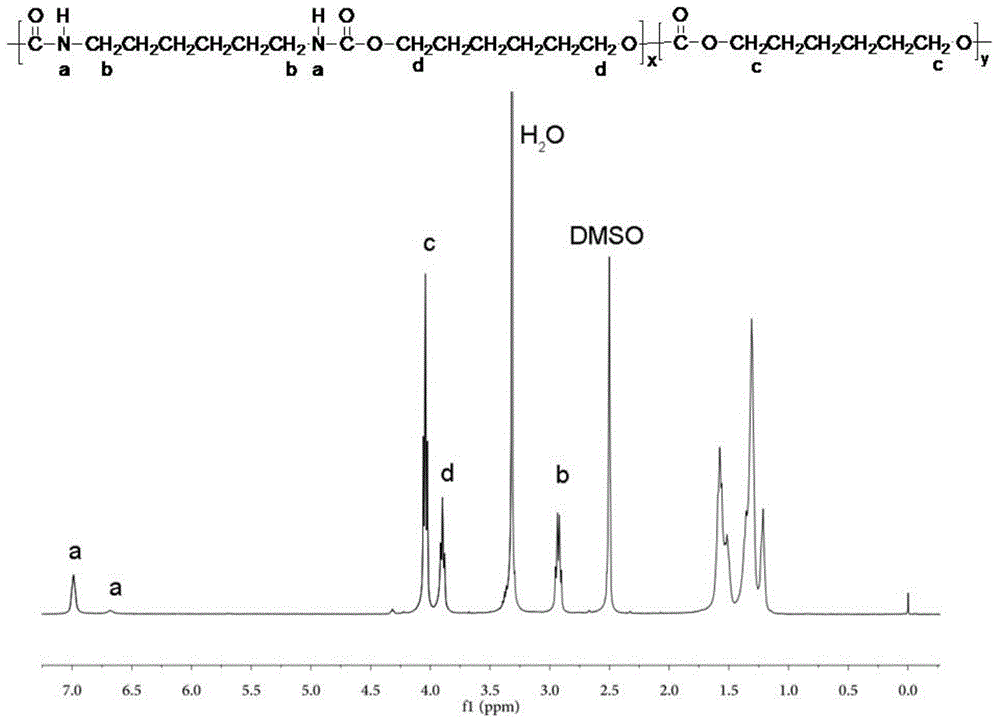
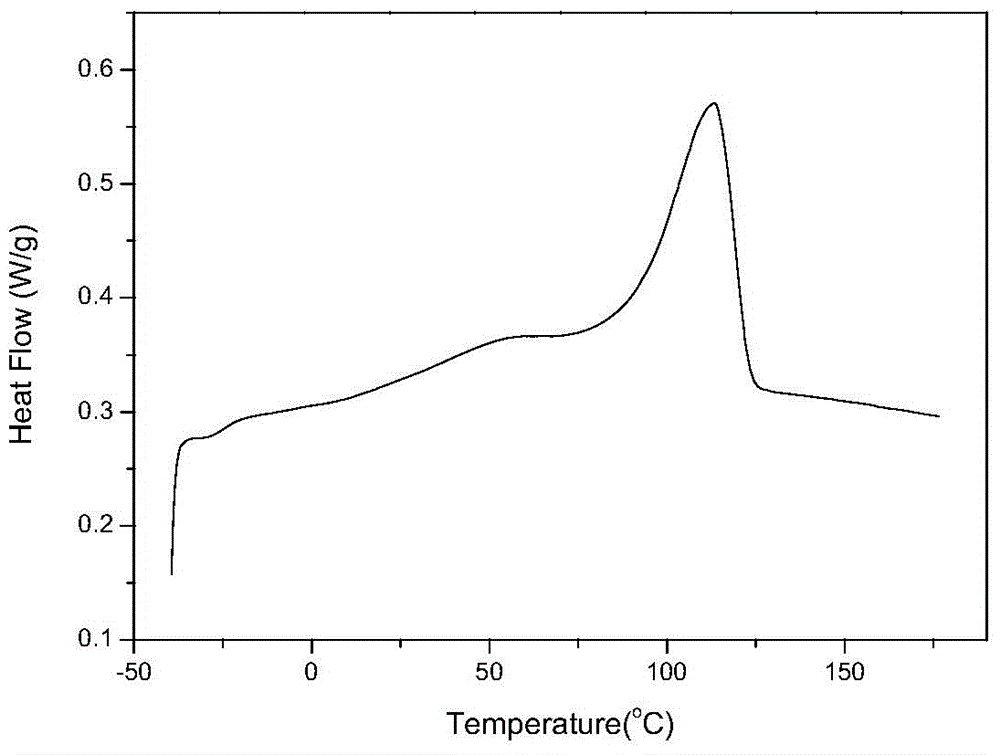
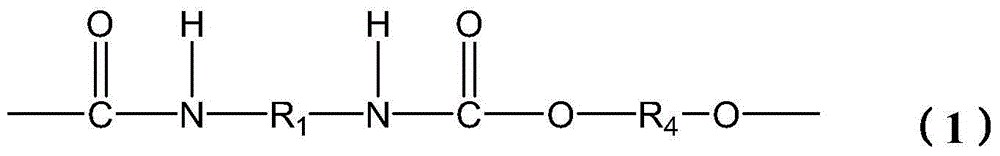
Polycarbonate polyurethane and green preparation method thereof
A polycarbonate type, polycarbonate technology, applied in the field of green preparation of the polyurethane, can solve the problems of difficulty in meeting performance requirements, low molecular weight of the product, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Take 34.8g of 1,6-hexamethylenediamine, 270g of dimethyl carbonate and 0.6g of catalyst sodium methoxide in the reaction kettle, feed nitrogen into the reaction kettle and install a condensation reflux device, raise the temperature to 75°C, and react for 2h After the reaction is completed, cool to room temperature, wash off the catalyst with excess dilute hydrochloric acid solution, filter with suction, and dry. Then the dried product is recrystallized by methanol, washed and dried to obtain white crystalline dimethyl carbamate monomer.
[0056] In an inert gas atmosphere, mix the above-mentioned dimethyl carbamate monomer, 88.5g 1,6-hexanediol and 3mg sodium carbonate, and carry out transesterification reaction at 160°C. The polycondensation reaction was carried out under the following conditions, and the hydroxyl-terminated polyurethane prepolymer was obtained after 2 hours of reaction.
[0057] The polyurethane prepolymer obtained above is mixed with 32.4 g of polyc...
Embodiment 2
[0059] Take 30g of 1,6-hexamethylenediamine, 145g of dimethyl carbonate and 0.8g of catalyst sodium methoxide in a reaction kettle, feed nitrogen into the reaction kettle and install a condensation reflux device, raise the temperature to 80°C, and react for 2h, After the reaction is completed, cool to room temperature, wash off the catalyst with excess dilute hydrochloric acid solution, filter with suction, and dry. Then the dried product is recrystallized by methanol, washed and dried to obtain white crystalline dimethyl carbamate monomer.
[0060] In an inert gas atmosphere, mix the above-mentioned dimethyl carbamate monomer, 72.5g of 1,6-hexanediol and 3.8mg of potassium carbonate, and carry out transesterification reaction at 180°C. The polycondensation reaction is carried out under pressure, and the hydroxyl-terminated polyurethane prepolymer is obtained after 2 hours of reaction.
[0061] The polyurethane prepolymer obtained above is mixed with 10.8g of polycarbonate di...
Embodiment 3
[0063] Take 42.2g of 1,4-butanediamine, 432g of dimethyl carbonate and 2.6g of catalyst sodium acetate in the reaction kettle, feed nitrogen into the reaction kettle and install a condensing reflux device, raise the temperature to 75°C, and react for 2h After the reaction is completed, cool to room temperature, wash off the catalyst with excess dilute hydrochloric acid solution, filter with suction, and dry. Then the dried product is recrystallized by methanol, washed and dried to obtain white crystalline dimethyl carbamate monomer.
[0064] In an inert gas atmosphere, mix the above-mentioned dimethyl carbamate monomer, 112g of 1,8-octanediol and 3mg of dibutyltin oxide, and carry out transesterification reaction at 165°C. The polycondensation reaction was carried out under the pressure, and the hydroxyl-terminated polyurethane prepolymer was obtained after 3 hours of reaction.
[0065] The polyurethane prepolymer obtained above is mixed with 15 g of polycarbonate diols with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com