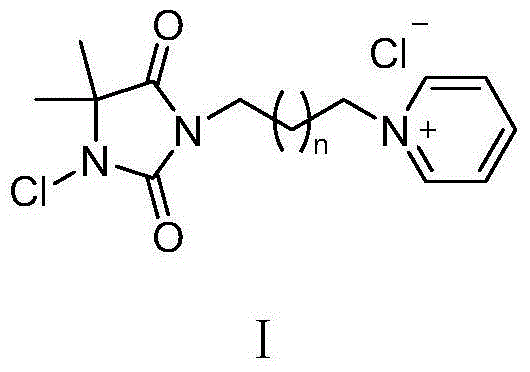
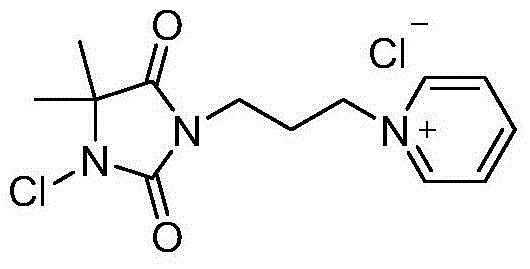
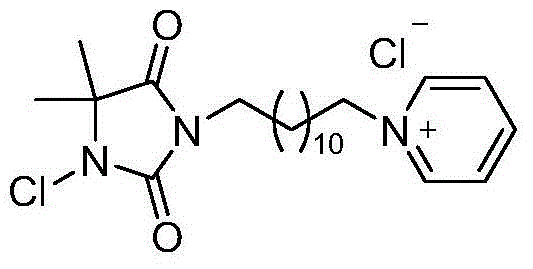
Pyridine quaternary ammonium salt type halamine antibacterial agent and preparation method thereof
A technology of pyridine quaternary ammonium salt and antibacterial agent, which is applied in the synthesis of pyridine quaternary ammonium salt compounds and the field of antibacterial, can solve problems such as poor water solubility of halamine antibacterial agent, avoid bacterial resistance, easily obtain raw materials, The effect of improving hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 5,5-dimethylhydantoin (3.026g, 23.6mmol) in 200mL acetone, stir to dissolve, add anhydrous K 2 CO 3 (12.050g, 87.2mmol), refluxed for 30min, then added 1,3-dibromopropane (7mL, 68.7mmol) to the mixture, continued to reflux for 4h to obtain a white suspension, and removed the solvent to obtain a white solid. Add 100mL of water and 100mL of ethyl acetate to it for extraction and liquid separation, take the upper layer liquid, remove the solvent and perform column chromatography, using ethyl acetate / petroleum ether as eluent. The resulting liquids containing the product were pooled, and the white solid obtained after removal of the solvent was the bromoalkylhydantoin compound (5.048 g, 85.8%).
[0023] Bromoalkylhydantoin compound (3.091g, 12.4mmol) was dissolved in 50mL of solvent acetonitrile, and excess pyridine (3.015mL, 37.4mmol) was added, heated to reflux overnight, and column chromatography was carried out after removing the solvent. Methane was used as ...
Embodiment 2
[0028] Dissolve 5,5-dimethylhydantoin (3.240g, 25.3mmol) in 200mL acetone, stir to dissolve, add anhydrous K 2 CO 3 (13.771g, 101.2mmol), refluxed for 30min, then added 1,3-dibromododecane (24.907g, 75.9mmol) to the mixture, continued to reflux for 24h to obtain a white suspension, and removed the solvent to obtain a white solid. Add 100mL of water and 100mL of ethyl acetate to it for extraction and liquid separation, take the upper layer liquid, remove the solvent and perform column chromatography, using ethyl acetate / petroleum ether as eluent. The resulting liquids containing the product were pooled, and the white solid obtained after removal of the solvent was the bromoalkylhydantoin compound (6.682 g, 70.4%).
[0029] Bromoalkylhydantoin compound (2.133g, 5.7mmol) was dissolved in 40mL of solvent acetonitrile, and excess pyridine (1.362g, 1.4mL, 17.1mmol) was added, heated to reflux overnight, and column chromatography was carried out after solvent removal, and methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com