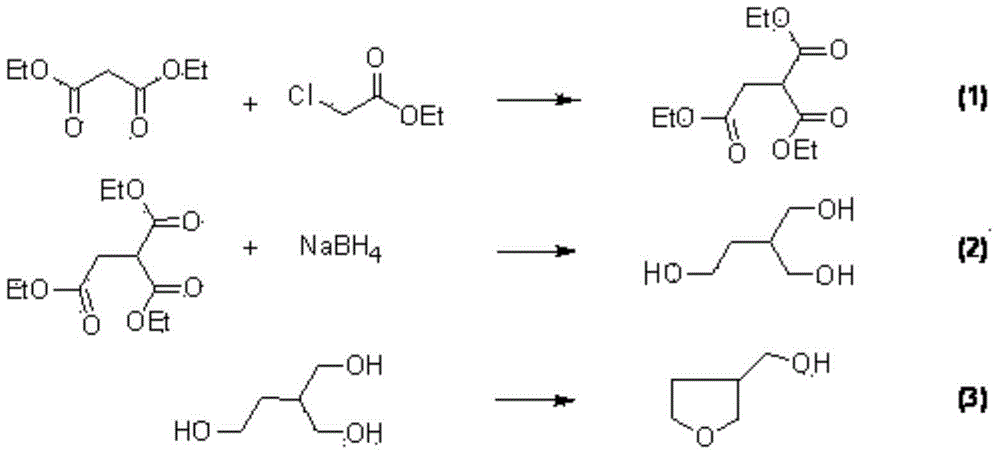
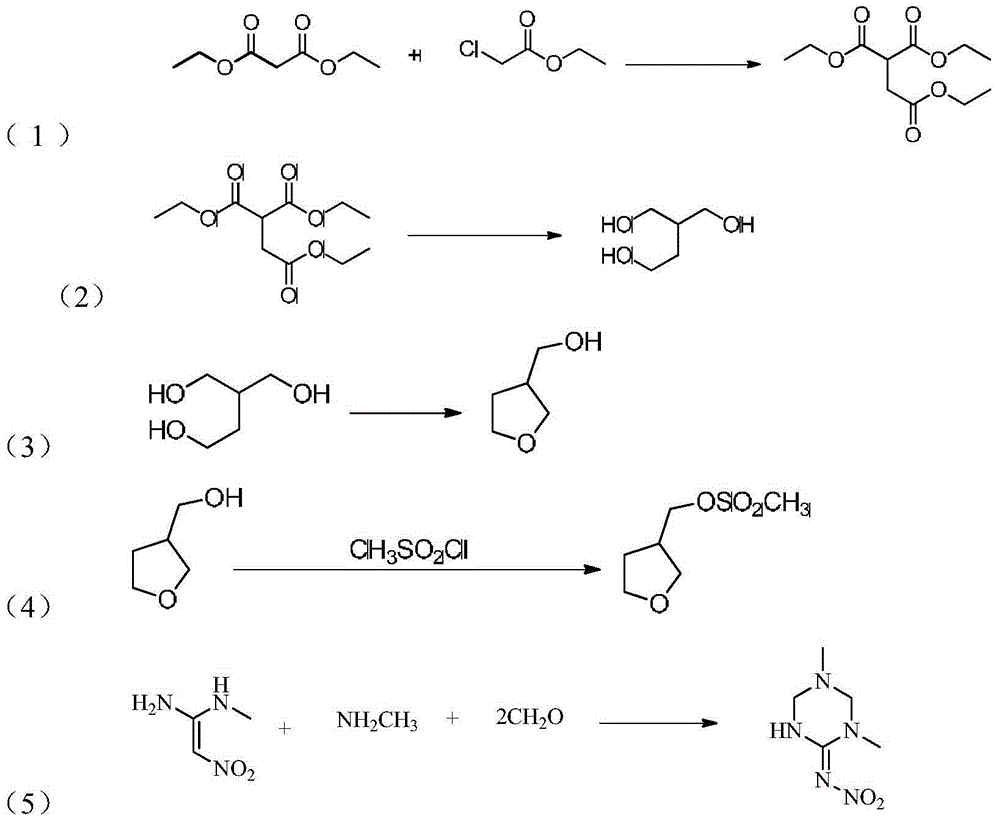
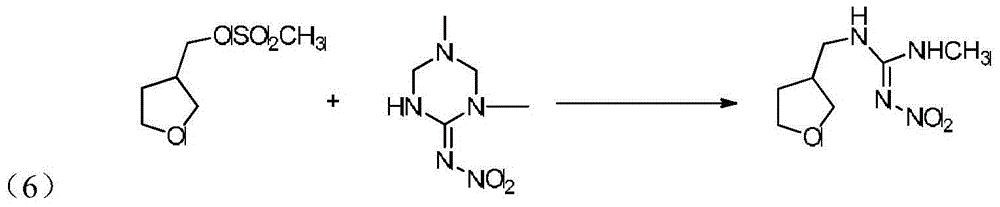
Synthesis method of dinotefuran
A synthesis method and technology of dinotefuran, applied in the field of synthesis of dinotefuran, can solve problems such as inability to produce on a large scale, high cost of raw materials, and difficulty in purification, and achieve the effects of easy operation, low consumption and high reaction selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Synthesis of ethyl ethane-1,1,2-tricarboxylate
[0034] Add 350ml of ethanol to the reaction flask, and add 20g of sodium tablets. An additional 128 g of diethyl malonate was added. After the addition, 88g of ethyl chloroacetate was added dropwise. After dropping, continue to reflux for 3 hours. After cooling down, adjust the pH value to 7, and filter with suction. Ethanol was removed by distillation, washed with water, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, and washed with anhydrous Na 2 SO 4 Dry and distill to obtain 200 g of crude ethane-1,1,2-tricarboxylic acid ethyl ester. The crude product was distilled under reduced pressure to obtain pure ethyl ethane-1,1,2-tricarboxylate.
[0035] Table 1 has provided the amount of all materials in the first step reaction in the embodiment.
[0036] Table 1
[0037] Material name
amount of material
350ml
Sodium tablets
20g
...
Embodiment 2
[0074] (1) Synthesis of α-ethoxycarbonyl-γ-butyrolactone
[0075]
[0076] Under the protection of nitrogen, 44g sodium metal was added to 900ml absolute ethanol, and stirred to dissolve. Under cooling with ice water, 320 g of diethyl malonate was added dropwise, and the mixture was stirred at room temperature for 0.5 h after the drop was completed. Then, a mixed solution prepared by 200 g of ethylene oxide and 300 ml of absolute ethanol was added dropwise, and the reaction temperature was controlled between 40°C and 45°C. After dropping, stir at room temperature for 15h. Then slowly add glacial acetic acid under ice water cooling, concentrate under reduced pressure and recover the solvent after adding, add 500mL of water and stir, separate the organic layer, dry it with anhydrous sodium sulfate and distill under reduced pressure to obtain the product α-ethoxycarbonyl-γ- Butyrolactone 150g.
[0077] The yield of the first step synthesis reaction is 80%, and the gas phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com