A kind of enrichment method and application of Sophora dihydroflavonoid G
A technology of dihydroflavonoids and Sophora genus, which is applied in the field of preparation of antitumor drugs, Sophora genus dihydroflavonoids G, can solve the problems of low content and low extraction yield, and achieve high purity and extraction yield of Sophora flavescens The effect of improving and enriching the method is simple and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1, the enrichment method of Sophora dihydroflavone G
[0036] 1) 500g of Sophora flavescens raw material, crushed and passed through No. 2 sieve, added 3000mL of ethyl acetate, ultrasonically extracted for 30min, filtered, added 3000mL of ethyl acetate to the residue, continued ultrasonically for 30min, filtered, combined filtrate, at a temperature lower than 50°C The organic solvent was recovered under reduced pressure to dryness to obtain 10.2 g of the total flavonoids sample of Sophora flavescens.
[0037] 2) Mix the total flavone solids of Sophora flavescens in step 1) with the newly prepared pyridine hydrochloride in a weight ratio of 1:1, N 2 Under protective conditions, the temperature was raised to 60°C for reaction. React for 4 hours, cool, add 100 mL of water, and freeze overnight at 4°C. Filtration, the solid is the crude product of Sophora flavone G.
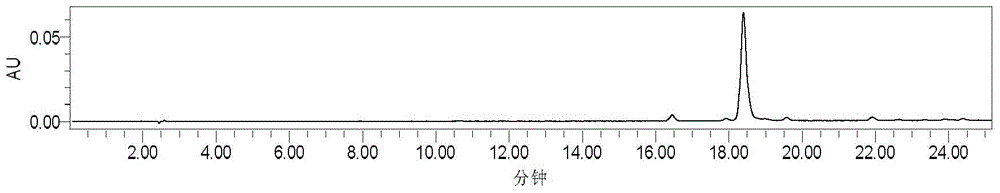
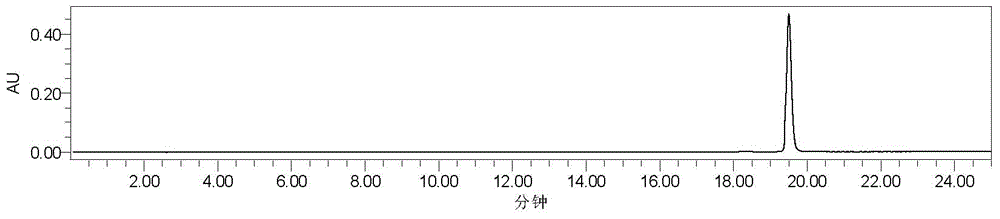
[0038] 3) The converted crude product is subjected to silica gel H column chromatography, and th...
Embodiment 2
[0041] Embodiment 2, the enrichment method of Sophora dihydroflavone G
[0042] 1) 500g of Sophora flavescens raw material, crushed and passed through No. 1 sieve, add 3000mL of 60% ethanol, ultrasonically extract for 60min, filter, add 60% ethanol to the residue, continue ultrasonic for 30min, filter, combine the filtrates, under the condition of lower than 50°C The organic solvent was recovered under reduced pressure to dryness, and 25.3 g of the total flavonoids of Sophora flavescens was obtained.
[0043] 2) Add 25.3g of the total flavonoids of Sophora flavescens in step 1) into a 1000mL dry four-necked reaction flask, add 10g of pyridine and 100g of methanol, heat up to 40°C, slowly add 5g of anhydrous aluminum trichloride in batches, add Complete the reaction at 50°C. TLC detection, stop reaction. Cool down to room temperature, add a small amount of saturated ammonium chloride aqueous solution until there is no solid in the reaction mixture, add ethyl acetate for extra...
Embodiment 3
[0047] Embodiment 3, the enrichment method of Sophora dihydroflavone G
[0048] 1) Sophora flavescens raw material 500g, crushed and passed through No. 2 sieve, add methanol 3000mL, ultrasonically extract for 60min, filter, add methanol to the residue and continue ultrasonically for 30min, filter, combine filtrate, and recover organic The solvent was dried to obtain 20.2 g of the total flavonoids of Sophora flavescens.
[0049] 2) Mix the 20.2g total flavonoids sample of Sophora flavescens in step 1) with the newly prepared pyridine hydrochloride in a weight ratio of 1:1, N 2 Under protective conditions, the temperature was raised to 50°C for reaction. React for 4 hours, cool, add 200 mL of water, and freeze at 4°C overnight. Filtration, the solid is the crude product of Sophora flavone G.
[0050] 3) performing silica gel column chromatography on Sophora dihydroflavone G, and eluting with petroleum ether-ethyl acetate system as the mobile phase system;
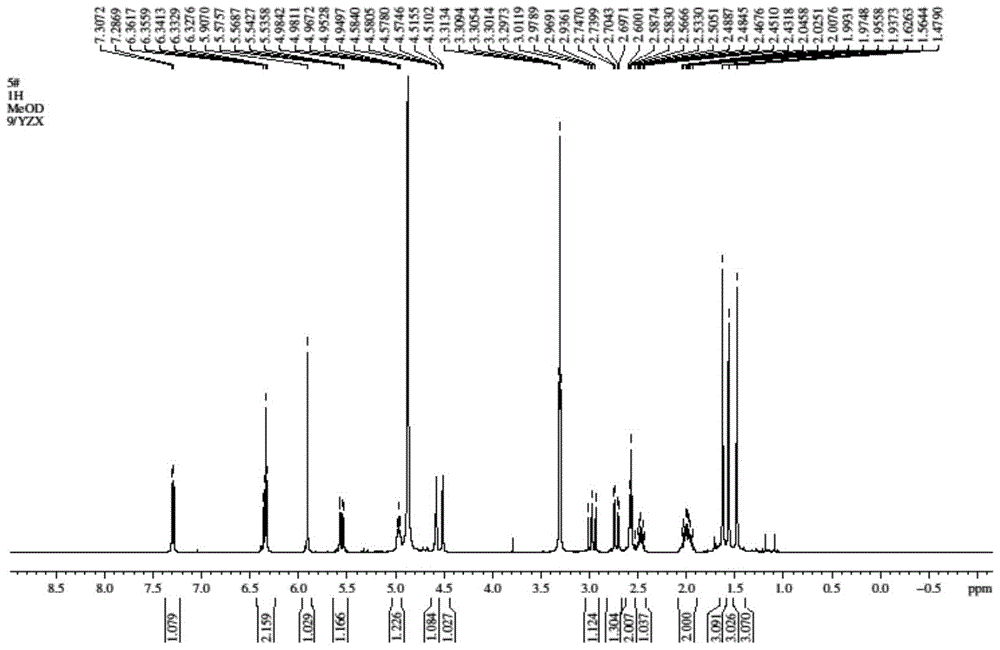
[0051] 4) Differe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com