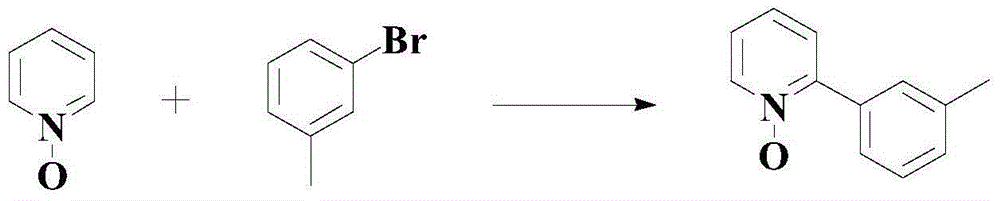
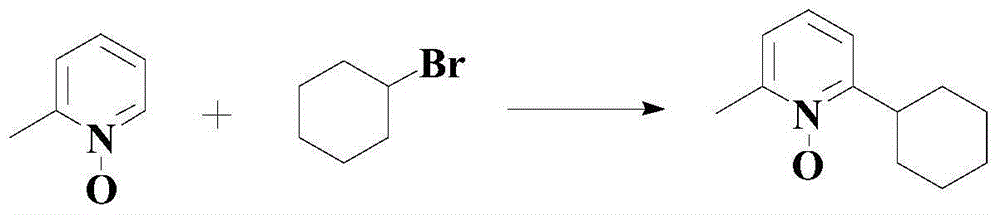
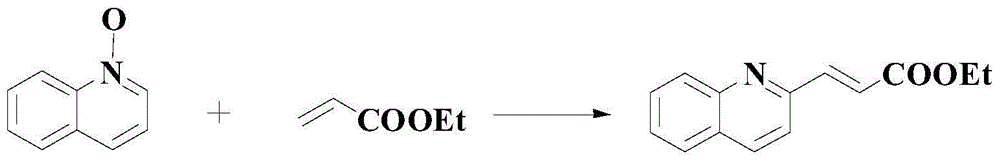Method for synthetizing medical intermediate heterocyclic group pyridine N-oxide
A technology of pyridine nitrogen oxide and synthesis method, which is applied in the synthesis of heterocyclic compounds and the synthesis field of heterocyclic substituted pyridine nitrogen oxides, and can solve the problems of low reaction yield and narrow application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Under a nitrogen atmosphere, add 100mmol formula (I) compound and 300mmol tetrahydrofuran to the reactor, then add 6mmol catalyst, 100mmol base triisopropanolamine, 200mmol oxidant cumene hydroperoxide and 5mmol auxiliary agent p-toluenesulfonic acid Sodium, stirred and heated to 80°C for 10 hours, cooled to room temperature after the reaction, added saturated brine, extracted three times with ether, combined organic phases, dried with anhydrous magnesium sulfate, concentrated in vacuo, passed through a 300-400 mesh silica gel column Chromatography, using a mixture of acetone and ethyl acetate at a volume ratio of 1:2 as the eluent, thereby obtaining the compound of formula (II) with a yield of 97.2%.
[0035] Among them, the catalyst is Fe(acac) 3 And the mixture of N-methyl-N-n-butylpyrrole bis(trifluoromethanesulfonyl)imide salt, where Fe(acac) 3 The molar ratio of N-methyl-N-n-butylpyrrole bis(trifluoromethanesulfonyl)imide salt is 1:0.1.
[0036] 1 H...
Embodiment 2
[0039]
[0040] Under a nitrogen atmosphere, add 100mmol formula (I) compound and 320mmol tetrahydrofuran to the reactor, then add 8mmol catalyst, 130mmol base triisopropanolamine, 250mmol oxidant cumene hydroperoxide and 7mmol auxiliary agent p-toluenesulfonic acid Sodium, stirred and heated to 90°C for 9 hours, cooled to room temperature after the reaction, added saturated brine, extracted three times with ether, combined organic phases, dried with anhydrous magnesium sulfate, concentrated in vacuo, passed through a 300-400 mesh silica gel column Chromatography, using a mixture of acetone and ethyl acetate at a volume ratio of 1:3 as the eluent, thereby obtaining the compound of formula (II) with a yield of 97.5%.
[0041] Among them, the catalyst is Fe(acac) 3 And the mixture of N-methyl-N-n-butylpyrrole bis(trifluoromethanesulfonyl)imide salt, where Fe(acac) 3 The molar ratio of N-methyl-N-n-butylpyrrole bis(trifluoromethanesulfonyl)imide salt is 1:0.15.
[0042] 1 H...
Embodiment 3
[0045]
[0046] Under a nitrogen atmosphere, add 100mmol formula (I) compound and 350mmol tetrahydrofuran to the reactor, then add 10mmol catalyst, 150mmol base triisopropanolamine, 300mmol oxidant cumene hydroperoxide and 8mmol auxiliary agent p-toluenesulfonic acid Sodium, stirred and heated to 100°C for 8 hours, cooled to room temperature after the reaction, added saturated brine, extracted three times with ether, combined organic phases, dried with anhydrous magnesium sulfate, concentrated in vacuo, passed through a 300-400 mesh silica gel column Chromatography, using a mixture of acetone and ethyl acetate at a volume ratio of 1:2 as the eluent, thereby obtaining the compound of formula (II) with a yield of 97.7%.
[0047] Among them, the catalyst is Fe(acac) 3 And the mixture of N-methyl-N-n-butylpyrrole bis(trifluoromethanesulfonyl)imide salt, where Fe(acac) 3 The molar ratio of N-methyl-N-n-butylpyrrole bis(trifluoromethanesulfonyl)imide salt is 1:0.2.
[0048] 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com