Purifying process for oral recombinant helicobacter pylori vaccine
A technology for Helicobacter pylori and vaccines, which can be used in antibacterial drugs, bacterial antigen components, chemical instruments and methods, etc., and can solve problems such as difficult preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
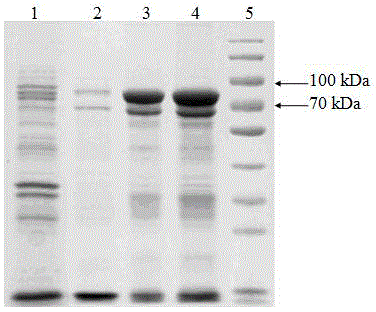
[0029] Cell fragmentation
[0030] Weigh 2000g of engineering bacteria, add 10mmol / L pH 8.0 Tris-HCl buffer solution at a ratio of 1:10, mix well, break the bacteria with a high-pressure homogenizer, break the bacteria three times at 700bar, and use a tubular centrifuge at 9000rpm Centrifuge at a flow rate of 1L / min, and the precipitate is the crude inclusion body. Microscopic examination of bacterial fragmentation rate ≥ 99%.
[0031] Washing and lysis of inclusion bodies
[0032] (1) Weigh 300g of inclusion bodies, add washing solution I at a ratio of 1:10, after the high-speed disperser is completely dispersed, mix and stir for 20min, centrifuge at 5000rpm for 40min, and collect the precipitate.
[0033] (2) Add washing liquid II to the precipitate, after the high-speed disperser is completely dispersed, mix and stir evenly, centrifuge at 5000rpm for 40min, and collect the precipitate.
[0034] (3) Add inclusion body lysate to the precipitate at a ratio of 1:10, stir overn...
Embodiment 2
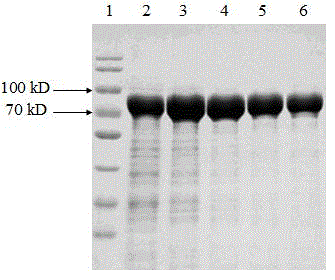
[0042] Cell fragmentation
[0043] Weigh 2000g of engineering bacteria, add 10mmol / L pH 6.0 Tris-HCl buffer solution at a ratio of 1:10, mix well, break the bacteria with a high-pressure homogenizer, break the bacteria twice at 600bar, and use a tubular centrifuge at 8000rpm Centrifuge at a flow rate of 1L / min, and the precipitate is the crude inclusion body. Microscopic examination of bacterial fragmentation rate ≥ 99%.
[0044] Washing and lysis of inclusion bodies
[0045] (1) Weigh 300g of inclusion bodies, add washing solution I at a ratio of 1:10, after the high-speed disperser is completely dispersed, mix and stir for 15min, centrifuge at 4000rpm for 20min, and collect the precipitate.
[0046] (2) Add washing liquid II to the precipitate, after the high-speed disperser is completely dispersed, mix and stir evenly, centrifuge at 4000rpm for 20min, and collect the precipitate.
[0047] (3) Add inclusion body lysate to the precipitate at a ratio of 1:10, stir overnight...
Embodiment 3
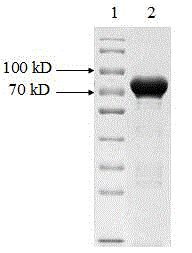
[0055] Cell fragmentation
[0056] Weigh 3000g of engineering bacteria, add 10mmol / L pH 9.0 Tris-HCl buffer solution at a ratio of 1:10, mix well and break the bacteria with a high-pressure homogenizer, break the bacteria three times at 800bar, and use a tubular centrifuge at 10000rpm Centrifuge at a flow rate of 2L / min, and the precipitate is the crude inclusion body. Microscopic examination of bacterial fragmentation rate ≥ 99%.
[0057] Washing and lysis of inclusion bodies
[0058] (1) Weigh 400g of inclusion bodies, add washing solution Ⅰ at a ratio of 1:10, after the high-speed disperser is completely dispersed, mix and stir for 30min, centrifuge at 6000rpm for 40min, and collect the precipitate.
[0059] (2) Add washing liquid II to the precipitate, after the high-speed disperser is completely dispersed, mix and stir evenly, centrifuge at 6000rpm for 40min, and collect the precipitate.
[0060] (3) Add inclusion body lysate to the precipitate at a ratio of 1:10, stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Protein concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Isoelectric point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com