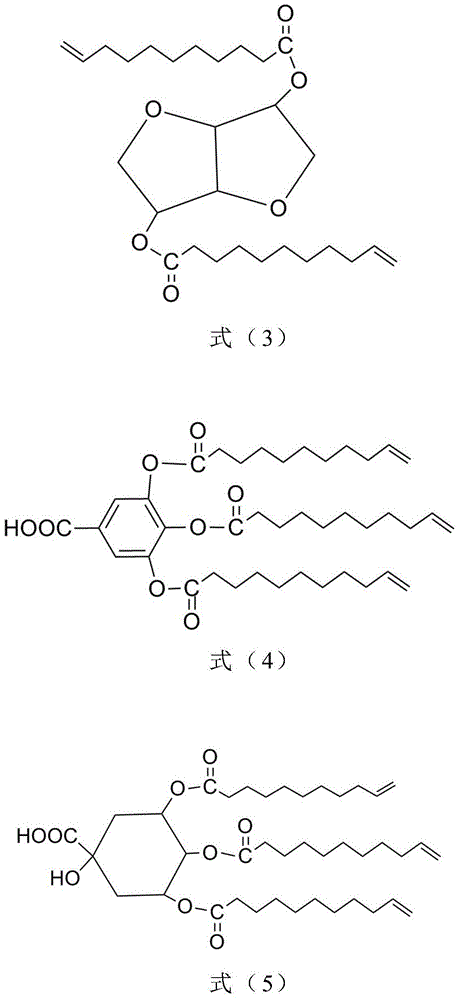
Method for preparing all-bio-based photocuring activated monomer and application of all-bio-based photocuring activated monomer
A technology of photoactive monomers and active monomers, which is applied in the field of preparation of all bio-based photocurable active monomers, can solve the problems of low renewable carbon content and lack of coating film performance, and achieve high reactivity, improved performance, The effect of alleviating dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
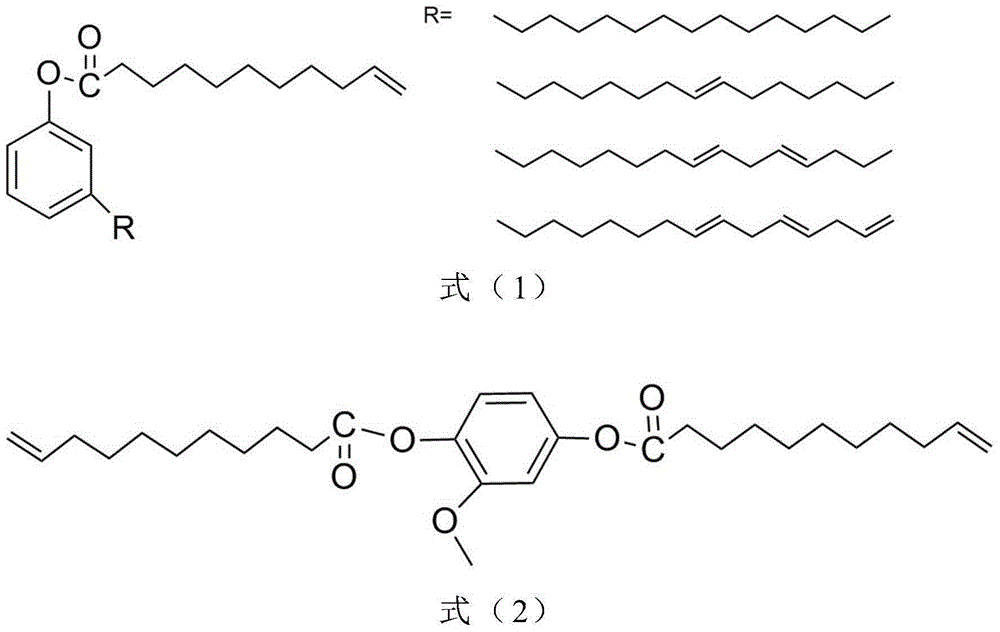
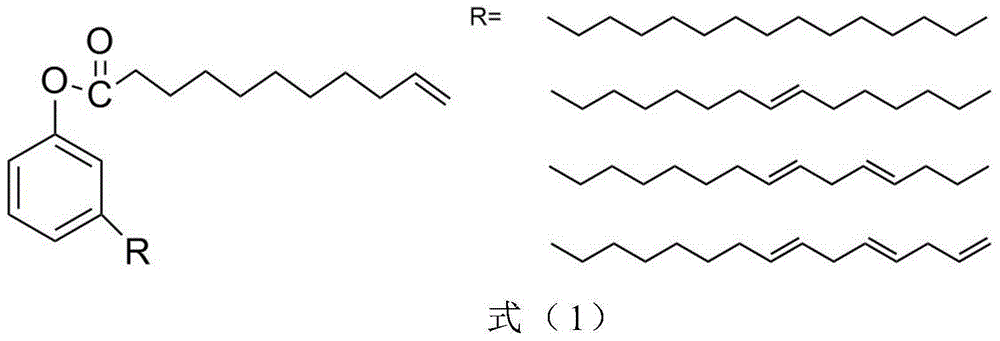
[0018] 1) Put 8g of vanillin, 110mL of tetrahydrofuran and 50mL of water into a two-necked bottle, fill it with nitrogen to protect, add 9.6g of sodium carbonate; react at room temperature for 5 hours, then add 0.1mol / L dilute hydrochloric acid to neutralize to PH=3 , THF was removed by rotary evaporation; the aqueous phase was extracted with ethyl acetate, the organic layer was washed with brine and dried with anhydrous sodium sulfate, filtered by suction and rotary evaporated to obtain vanillin-based diol.
[0019] 2) Put 2.0 g of vanillin-based diol, 4.3 g of triethylamine, and 10 g of ethyl acetate in a 100 mL flask, and add 8.6 g of undecylenyl chloride dropwise into the flask with a constant pressure dropping funnel under an ice bath. The reaction was first reacted in an ice bath for 2 hours, then warmed up to room temperature and reacted for 24 hours, filtered with suction, the liquid phase was washed with 1% aqueous sodium hydroxide solution and distilled water, dried w...
Embodiment 2
[0023] Take 2g of cardanol, 1g of triethylamine and 10g of ethyl acetate respectively in a 100mL flask, and add 2g of undecylenoyl chloride dropwise into the flask with a constant pressure dropping funnel under ice bath. The reaction was first reacted in an ice bath for 2 hours, then warmed up to room temperature and reacted for 24 hours, filtered with suction, and the liquid phase was washed with 1% aqueous sodium hydroxide solution and distilled water, dried with anhydrous magnesium sulfate, filtered with suction and rotary steamed to obtain cashew nuts Phenolic reactive monomers. (yield: 87%)
[0024] 1 H NMR (400MHz, CDCl 3 )δ7.30(s,1H),7.07(s,1H),6.92(s,2H),5.84(td,J=16.8,6.9Hz,2H),5.39(s,2H),5.00(s,4H ), 2.59(s,4H), 2.30(s,2H), 2.07(s,4H), 1.78(s,2H), 1.64(s,4H), 1.34(s,28H), 0.91(s,2H).
[0025] 13 C NMR (101MHz, CDCl 3 )δ171.13 (COO), 149.70 (C), 143.90 (C), 138.12 (CH 2 =CH),129.23(CH),128.92(CH),128.03(CH),124.76(CH),120.37(CH),117.68(CH 2 =CH),113.14(CH 2 =...
Embodiment 3
[0027] Take 1.0 g of gallic acid, 5.4 g of undecylenoyl chloride and 10 g of acetone in a 100 mL flask, and add 2.7 g of triethylamine dropwise into the flask with a constant pressure dropping funnel under ice-cooling. The reaction was first reacted in ice bath for 2h, then warmed up to room temperature and reacted for 24h, filtered with suction, and the acetone solvent was removed by rotary evaporation, and ethyl acetate was used as the solvent instead. Then wash with 1% aqueous sodium hydroxide solution and distilled water respectively, dry with anhydrous magnesium sulfate, filter with suction and rotary evaporate to obtain gallic acid-based active monomer. (yield: 78%)
[0028] 1 H NMR (400MHz, Acetone-D 6 )δ7.68(s,2H),5.68(s,3H),4.83(s,6H),3.44(s,3H),2.46(s,4H),2.14(s,2H),1.91(s,8H ),1.58(s,6H),1.22(s,32H),0.99(s,6H).
[0029] 13 C NMR (101MHz, Acetone-D 6 )δ170.28 (COO), 168.93 (COOH), 143.22 (C), 139.71 (CH 2 =CH), 138.93(C), 122.09(C), 118.11(CH), 113.84(CH 2 =C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com