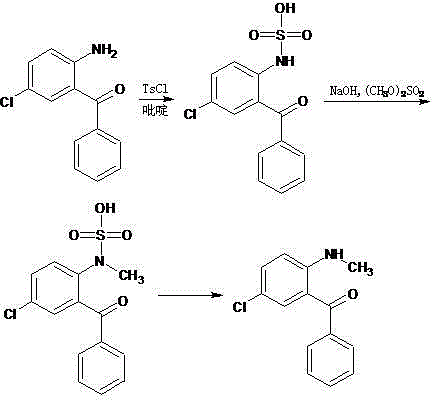
Preparation method of 2-methylamino-5-chlorobenzophenone
A technology of chlorobenzophenone and methylamino, which is applied in the field of medicine and chemical industry, can solve the problems of difficult to stir, stop the reaction, and high cost of solvents, achieve good structural stability, reduce additional costs, and avoid toxicity hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
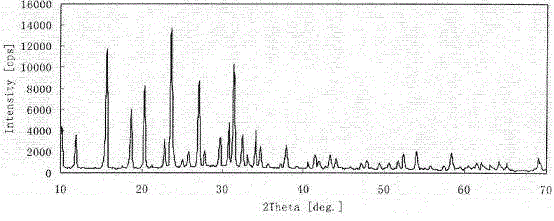
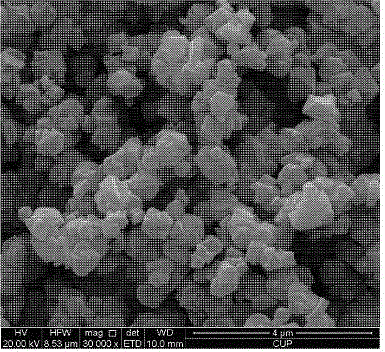
[0035] Preparation of NaY Molecular Sieve with High Si-Al Ratio and Small Grain
[0036] The hydrothermal synthesis method is used to synthesize NaY molecular sieves with high silicon-aluminum ratio and small crystal grains by increasing the ratio of silicon to aluminum in the framework on the basis of controlling the grain size. The molecular sieve has good structural stability and catalytic activity, and the preparation method has easy-to-obtain raw materials, simple operation, low production cost and no environmental pollution, and has industrial application prospects.
[0037] The preparation method of NaY molecular sieve with high silicon-aluminum ratio and small grain size:
[0038] 21g sodium hydroxide and 7.2g sodium aluminate (ωAl 2 o 3 )≥41.0 %) was dissolved in 80g deionized water, stirred until clear, then added 125g water glass (ω(Na 2 O) = 6.98 %; ω(SiO 2 ) = 19.68 %), the directing agent was obtained by rotating and dynamically aging at 80°C for 12 h and at ...
Embodiment 1
[0046] Weigh 231g of 2-amino-5-chlorobenzophenone, 900g of dimethyl carbonate and 70g of NaY molecular sieve with high silicon-aluminum ratio and small crystal grains, and place them in a reaction flask. Stir and heat to 80°C. After the heat preservation reaction for 8 hours, the sample was analyzed, and the content of the raw material 2-amino-5-chlorobenzophenone in the reaction solution was found to be 0.2%, and the reaction could be stopped.
[0047] Pass water to cool to room temperature, remove the catalyst by filtration, transfer the filtrate to a decompression flask, distill off the unreacted dimethyl carbonate under reduced pressure, recrystallize the residue with ethanol to obtain yellow needle crystals, and obtain 2-methylamino -5-Chlorobenzophenone 221 g, yield 90.2%, melting point 94.0-95.1°C, HPLC purity 98.9%.
Embodiment 2
[0049] Weigh 231g of 2-amino-5-chlorobenzophenone, 900g of dimethyl carbonate and 186g of NaY molecular sieve with high silicon-aluminum ratio and small crystal grains, and place them in a reaction flask. Stir and heat to 90°C. After 6.5 hours of heat preservation reaction, sampling and analysis showed that the content of the raw material 2-amino-5-chlorobenzophenone in the reaction solution was 0.3%, and the reaction could be stopped.
[0050] Pass water to cool to room temperature, remove the catalyst by filtration, transfer the filtrate to a decompression flask, distill off the unreacted dimethyl carbonate under reduced pressure, recrystallize the residue with ethanol to obtain yellow needle crystals, and obtain 2-methylamino -5-Chlorobenzophenone 225 g, yield 91.8%, melting point 94.2-95.1°C, HPLC purity 98.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com