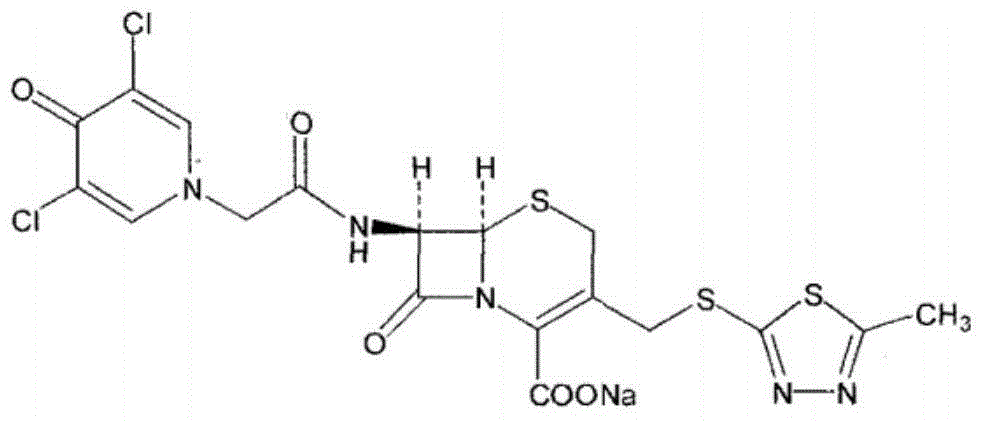
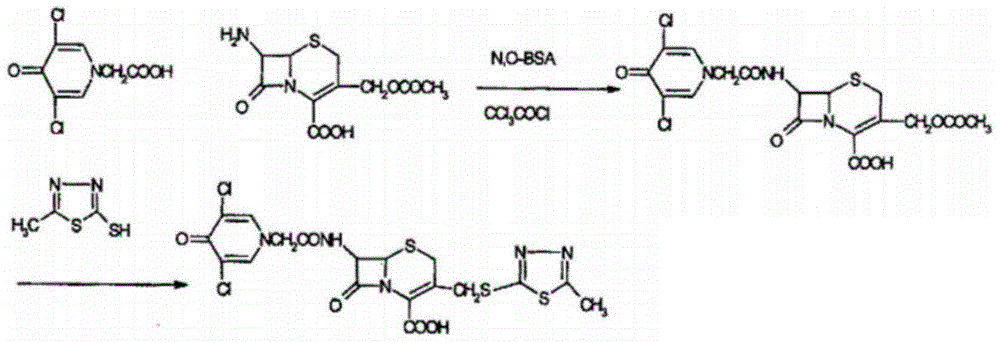
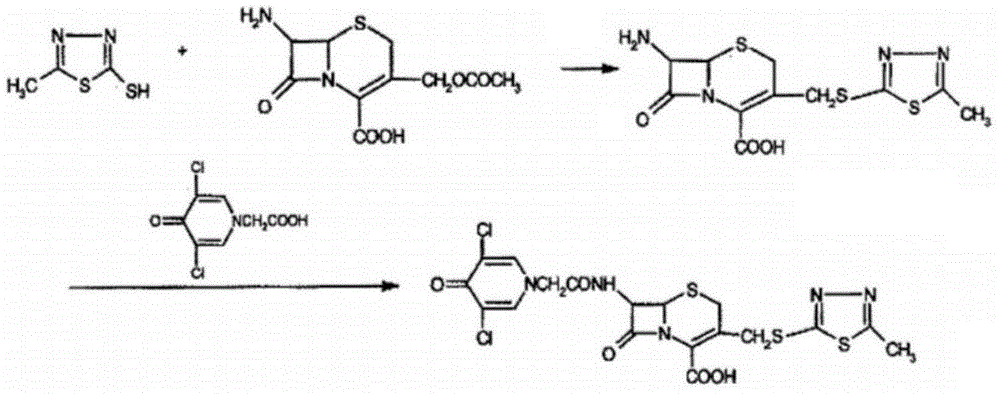
Enzymatic synthesis technology of novel cephalo-type anti-infection drug
A technology of enzymatic synthesis and cephalosporins, applied in the direction of fermentation, can solve the problems of unsuitability for industrial production, difficult to find purification methods, and high industrial production costs, and achieve stable and reliable reactions, high product quality, and little environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of TDA hydrochloride (TDA·HCl):
[0034] Add 36.5g TDA (Shanghai Yifei Biotechnology Co., Ltd., content: 94.5%, 0.1mol) to 180ml dimethylformamide, stir for 10min, slowly add 20ml of concentrated hydrochloric acid dropwise, finish adding in 30min, adjust the temperature to 25~ React at 30°C for 2 hours; after the reaction, slowly lower the temperature to below 10°C to crystallize for 2 hours, filter, and wash the filter cake with 70ml of acetone for 2 times, and dry it in vacuum at 30-35°C for 3-4 hours to obtain 37.4g of TDA hydrochloride ( TDA·HCl, 0.097mol,), the content is 99.8%, and the molar yield is 98%.
[0035] (2) Preparation of TDA sodium salt (TDA-Na):
[0036]Put 37.4 g of TDA hydrochloride (TDA·HCl, 0.097 mol) obtained in step (1) into a reaction flask, add 160 ml of water, add 17.0 g of sodium bicarbonate in batches under stirring at room temperature to dissolve the solid, filter the reaction solution, and add the filtrate to 300ml of ac...
Embodiment 2
[0040] (1) Preparation of TDA hydrochloride (TDA·HCl):
[0041] Add 38g TDA (Shanghai Yifei Biotechnology Co., Ltd., content 90.3%, 0.1mol) to 200ml isopropanol, stir for 10min, slowly add 38ml concentrated hydrochloric acid dropwise, finish adding in 30min, adjust the temperature to 25~30℃ for reaction 2 hours; after the reaction was completed, the temperature was slowly lowered to below 10°C to crystallize for 1 hour, filtered, and the filter cake was beaten and washed with 70ml of acetone twice, and vacuum-dried at 30-35°C for 3-4 hours to obtain 36.9g of TDA hydrochloride (TDA·HCl , 0.0965mol,), the content is 99.6%, and the molar yield is 96.5%.
[0042] (2) Preparation of TDA sodium salt (TDA-Na):
[0043] Put 36.9 g of TDA hydrochloride (TDA·HCl, 0.0965 mol) obtained in step (1) into a reaction flask, add 200 ml of water, add 17 g of sodium bicarbonate in batches under stirring at room temperature to dissolve the solid, filter the reaction solution, and add the filtrat...
Embodiment 3
[0047] (1) Preparation of TDA hydrochloride (TDA·HCl):
[0048] Add 35g of TDA (Suzhou Vita Chemical Co., Ltd., content: 98.4%, 0.1mol) to 200ml of methanol, stir for 10min, slowly add 18ml of concentrated hydrochloric acid dropwise, finish adding in 30min, adjust the temperature to 30-35°C and react for 1.5 hours; After the reaction is completed, slowly lower the temperature to below 10°C to crystallize for 2 hours, filter, and wash the filter cake with 70ml of acetone twice, and dry it in vacuum at 30-35°C for 3-4 hours to obtain TDA hydrochloride (TDA·HCl, 0.0965mol,) 37g, the content is 99.8%, and the molar yield is 97%.
[0049] (2) Preparation of TDA sodium salt (TDA-Na):
[0050] Put 37g of TDA hydrochloride (TDA·HCl, 0.0965mol) obtained in step (1) into a reaction flask, add 200ml of water, add 17g of sodium bicarbonate in batches under stirring at room temperature to dissolve the solid, filter the reaction solution, add acetone to the filtrate 350ml until turbid, st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com