Method for determining aureomycin premix content and related substances through high-performance liquid chromatography method
A technology of high-performance liquid chromatography and related substances, which is applied in the field of high-performance liquid chromatography to determine the content of aureomycin premix and related substances, can solve the problems of harsh mobile phase, many influencing factors, and high testing cost, and achieve good linearity Correlation, high test accuracy, good maintenance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] The preferred embodiments of the present invention will be described in detail below with reference to the accompanying drawings.
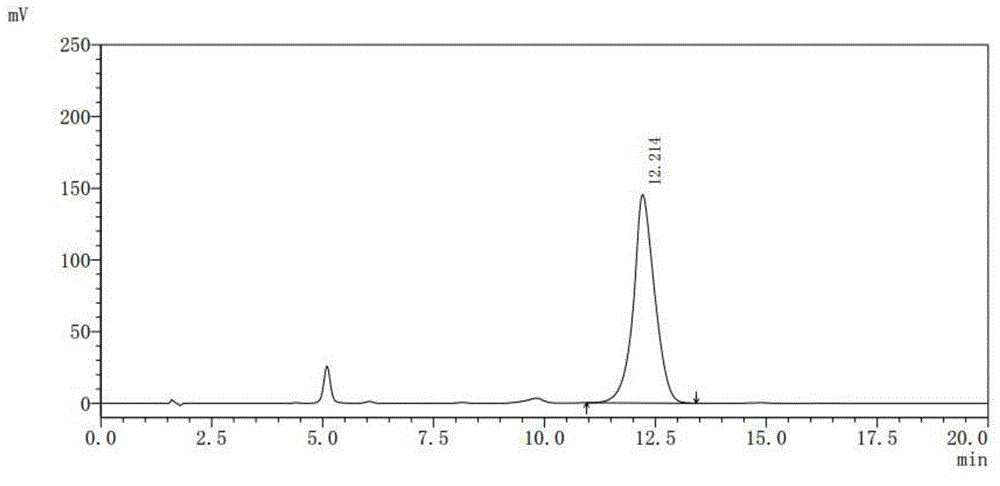
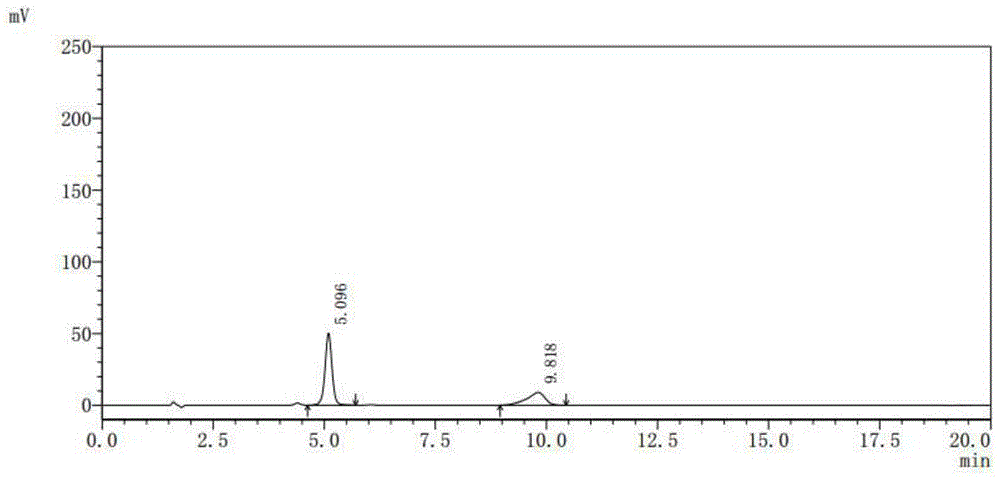
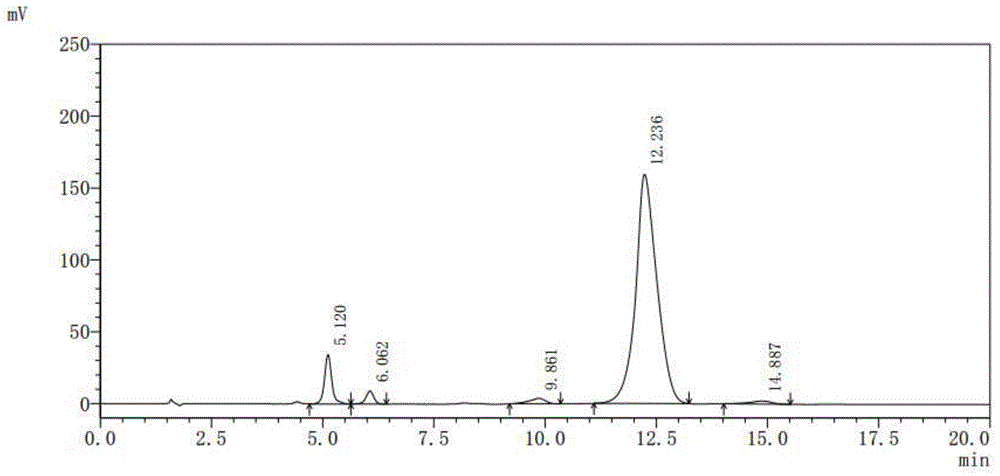
[0039] Testing instruments and conditions:
[0040] Shimadzu LC-20 series high performance liquid chromatography, SPD-M20A detector;
[0041] Chromatographic column: C18, 5μ, 4.6×150mm;
[0042] Column temperature control: 35°C;
[0043] Detection wavelength: UV 365nm;
[0044] Mobile phase: water: 2mol / L phosphoric acid solution: N,N-dimethylformamide = 70:2:28 (volume ratio);
[0045] Pump flow: 1.00ml / min;
[0046] Quantitative volume: 20μL;
[0047] Purified water (HPLC grade), hydrochloric acid (excellent grade), phosphoric acid (excellent grade), N,N-dimethylformamide (chromatographic grade)
[0048] Preparation of mobile phase solution: Take 700ml of purified water, add 20ml of 2mol / L phosphoric acid solution and mix well, then add 280ml of N,N-dimethylformamide, filter through a 0.45μm membrane with a vacuum filter, and set as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com