Semicarbazone compound preparation method and application in biomedicine
A compound and solvate technology, applied in the chemical field, can solve the problems of enrichment secondary damage, chelation ability destroying the homeostasis of copper ions, and difficulty in metabolism.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
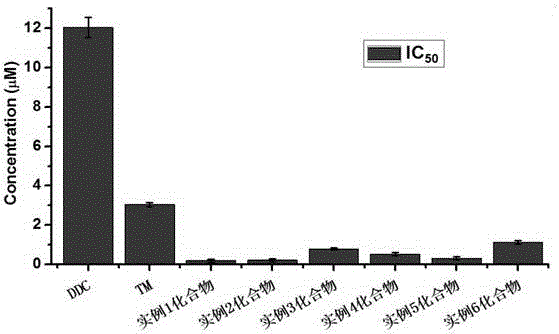
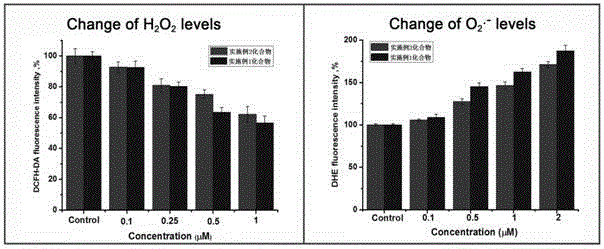
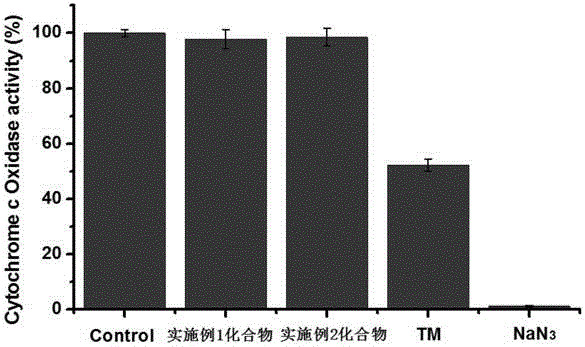
Image
Examples
Embodiment 1
[0066] Compound 1:
[0067] Preparation of (1,5)-1-(5-chloro-2-hydroxybenzylidene)-5-((4-oxo-4H-chromen-3-yl)methylene)thiocarbonohydrazide
[0068]
[0069] The reaction equation is as follows:
[0070]
[0071] Step 1: Dissolve excess thiocarbazide (30 mmol, 3.18 g,) in 100 mL of ethanol / water mixed solvent (50% by volume) at room temperature, and heat to 60-90° C. to dissolve completely. Then, an ethanol solution of 5-chlorosalicylaldehyde (10 mmol, 1.57 g) was slowly added dropwise to the above solution using a constant pressure dropper. A catalytic amount of glacial acetic acid was added dropwise and reacted at a constant temperature for 1-2 hours to obtain a white insoluble matter, which was filtered by suction, collected, rinsed with ethanol and deionized water, and dried.
[0072]Step 2, phosphorus oxychloride (0.3mol, 46.0g) is slowly added dropwise in 60mL DMF under ice bath condition, then the DMF solution of o-hydroxyacetophenone (0.1mol, 13.60g) is lower t...
Embodiment 2
[0075] Compound 2
[0076] Preparation of (1,5)-1-(2-hydroxybenzylidene)-5-((4-oxo-4H-chromen-3-yl)methylene)thiocarbonohydrazide
[0077]
[0078] The reaction equation is the same as shown in Example 1, and the corresponding raw material is replaced by salicylaldehyde.
[0079] Step 1: Dissolve excess thiocarbazide (3.18 g, 30 mmol) in 100 mL of ethanol / water mixed solvent (50% by volume) at room temperature, and heat to 60-90° C. to completely dissolve. A solution of salicylaldehyde (1.22 g, 10 mmol) in ethanol (10 mL) was slowly added dropwise to the above solution using a constant pressure dropper. A catalytic amount of glacial acetic acid was added dropwise and reacted at a constant temperature for 1-2 hours to obtain a light yellow insoluble matter, which was collected by suction filtration, rinsed with ethanol and deionized water, and dried.
[0080] Step 2, dissolve the aldehyde structure compound (10mmol, 1.74g) of the intermediate described in formula (II) in 2...
Embodiment 3
[0082] Compound 3
[0083] Preparation of (1,5)-1-((4-oxo-4H-chromen-3-yl)methylene)-5-((pyridin-2-yl)methylene)thiocarbonohydrazide
[0084]
[0085] The reaction equation is the same as shown in Example 1, and the corresponding raw material is replaced by 2-pyridinecarbaldehyde.
[0086] Step 1: Dissolve excess thiocarbazide (30 mmol, 3.18 g) in 100 mL of ethanol / water mixed solvent (50% by volume) at room temperature, and heat to 80° C. to completely dissolve. A solution of 2-pyridinecarbaldehyde (10 mmol, 1.07 g) in ethanol (10 mL) was slowly added dropwise to the above solution using a constant pressure dropper. A catalytic amount of glacial acetic acid was added dropwise and reacted at a constant temperature for 1 h to obtain a light yellow insoluble matter, which was collected by suction filtration, rinsed with ethanol and deionized water, and dried.
[0087] Step 2, dissolve the intermediate compound (10mmol, 1.74g) described in formula (II) in 20mL DMSO, add the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com