Preparation method for layered double hydroxide (LDH) nanoscrolls
A layered bimetallic, hydroxide technology, applied in chemical instruments and methods, nanotechnology, nickel compounds, etc., can solve the problems of high reaction temperature, high energy consumption and danger, achieve large specific surface area, avoid agglomeration, The effect of less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In 11 mL of n-hexanol, add 11 g of cetyltrimethylammonium bromide and stir to dissolve. Add 50mL of n-hexane into the system and stir for 30min to obtain a uniform and stable emulsion suspension system.
[0046] In 5 mL deionized water, add 0.105 g CoSO 4 with 0.04gAl 2 (SO 4 ) 3 , stirred to dissolve and added dropwise to the above suspension system. In 5 mL of deionized water, add 0.27 g of urea, dissolve it and add it dropwise to the above system and keep stirring for 1 h to obtain a clear and transparent microemulsion system.
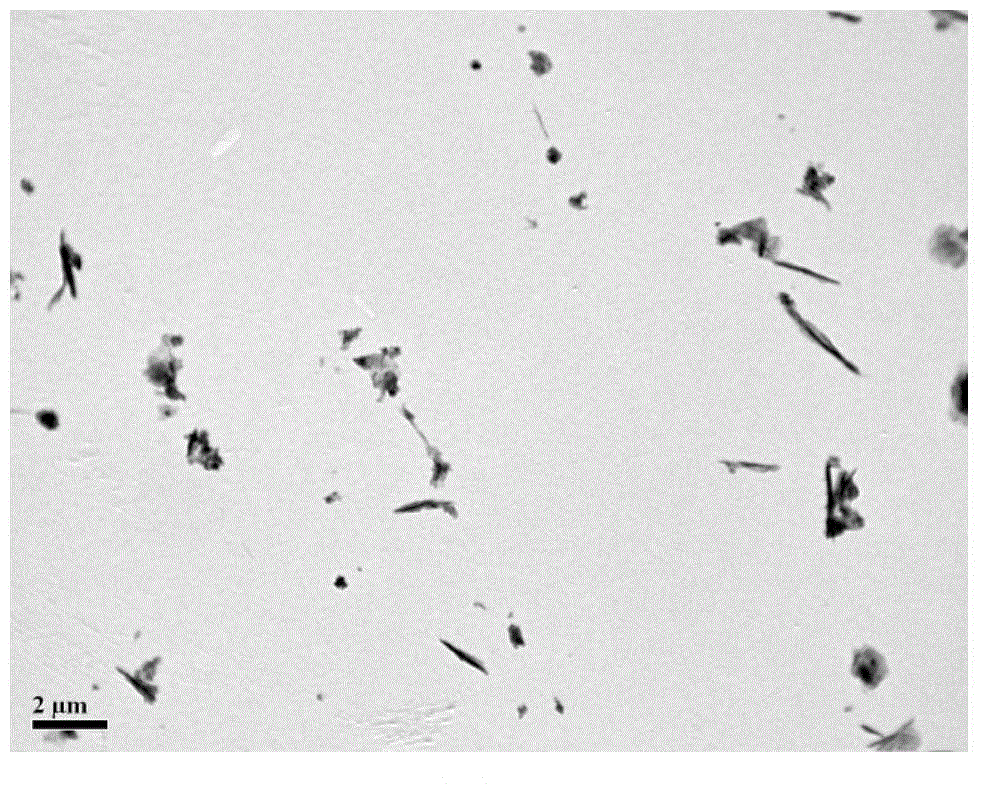
[0047] The above-mentioned microemulsion system was transferred to a hydrothermal kettle, and placed in a vacuum oven for crystallization at 95° C. for 12 hours. After the reaction was completed, it was taken out after cooling down to room temperature, washed several times by centrifugation with ethanol and deionized water respectively, and finally freeze-dried to obtain a layered double metal hydroxide.
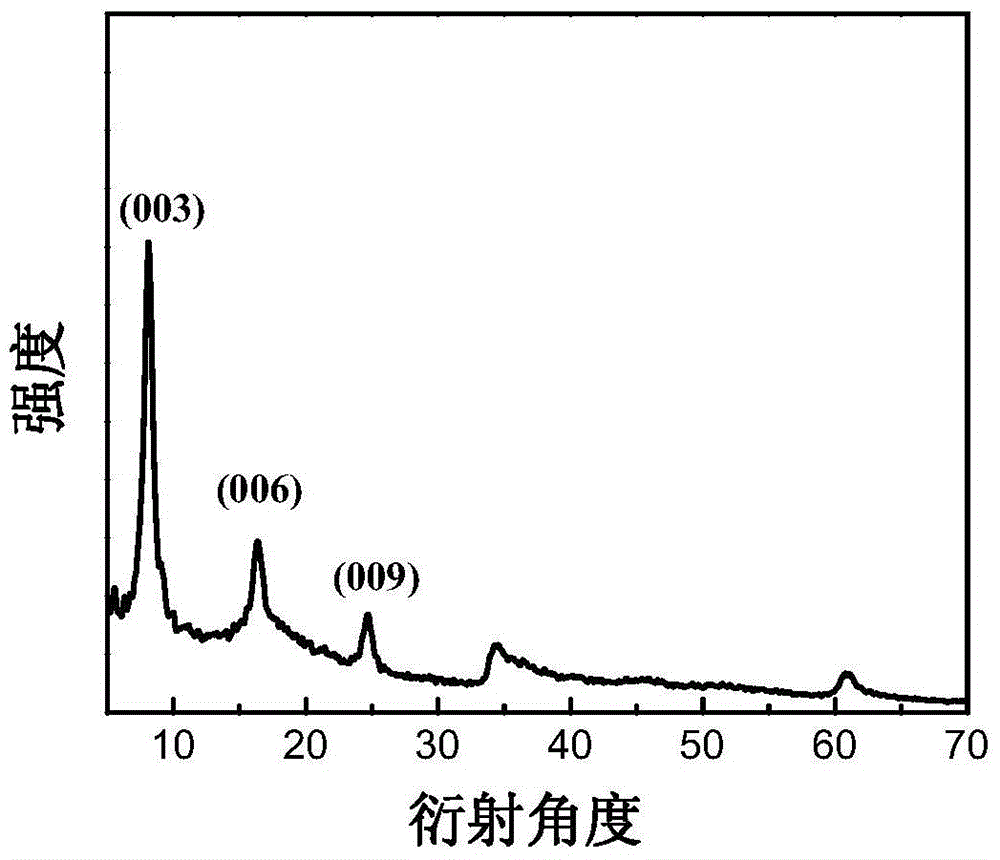
[0048] The XRD spectrogram of ...
Embodiment 2
[0051] In 11 mL of n-hexanol, add 11 g of cetyl dimethyl benzyl ammonium chloride and stir to dissolve. Add 50mL of n-hexane into the system and stir for 30min to obtain a uniform and stable emulsion suspension system.
[0052] In 5 mL deionized water, add 0.105 g CoSO 4 with 0.04gAl 2 (SO 4 ) 3 , stirred to dissolve and added dropwise to the above suspension system. In 5 mL of deionized water, add 0.27 g of urea, dissolve it and add it dropwise to the above system and keep stirring for 3 hours to obtain a clear and transparent microemulsion system.
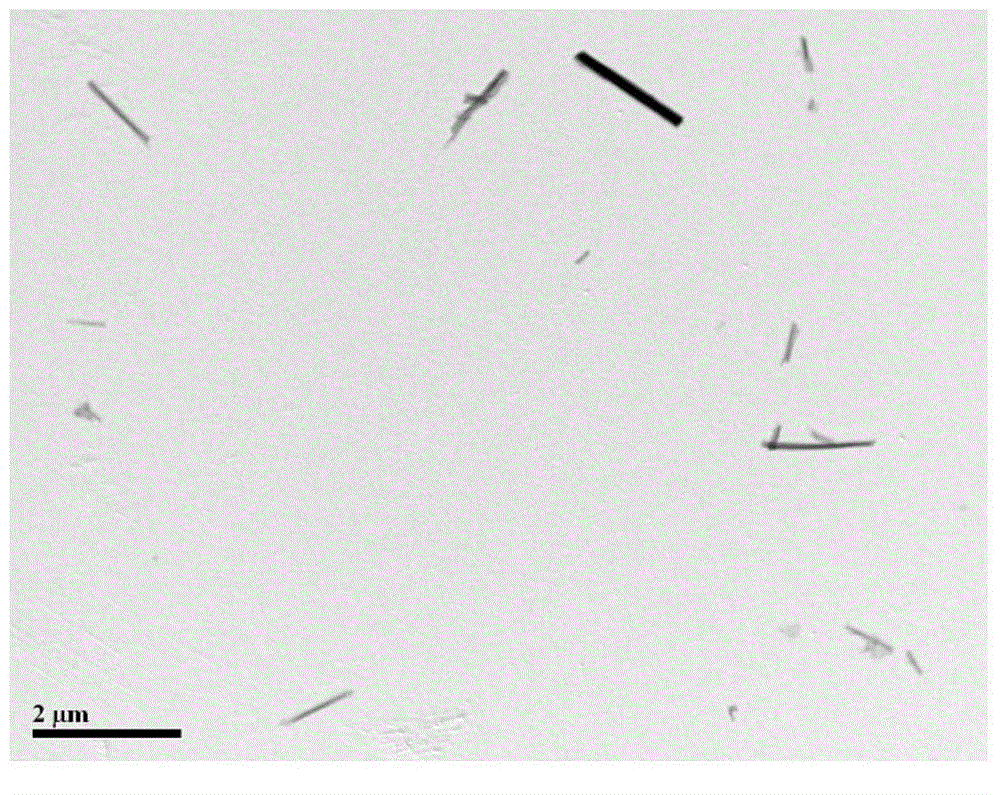
[0053] The above-mentioned microemulsion system was transferred to a hydrothermal kettle, and placed in a vacuum oven for crystallization at 110° C. for 15 hours. After the reaction was completed, it was taken out after cooling down to room temperature, washed several times by centrifugation with ethanol and deionized water respectively, and finally freeze-dried to obtain a layered double metal hydroxide.
[0054] The XRD s...
Embodiment 3
[0057] In 11 mL of n-hexanol, add 12 g of tetradecyldimethylbenzyl ammonium bromide and stir to dissolve. Add 50mL of n-hexane into the system and stir for 30min to obtain a uniform and stable emulsion suspension system.
[0058] In 15 mL deionized water, add 0.105 g CoSO 4 with 0.04gAl 2 (SO 4 ) 3 , stirred to dissolve and added dropwise to the above suspension system. In 5 mL of deionized water, add 0.27 g of urea, dissolve it, add it dropwise to the above system and keep stirring for 1 h to obtain a clear and transparent microemulsion system.
[0059] The above-mentioned microemulsion system was transferred to a hydrothermal kettle, and placed in a vacuum oven for crystallization at 125° C. for 30 h. After the reaction was completed, it was taken out after cooling down to room temperature, washed several times by centrifugation with ethanol and deionized water respectively, and finally freeze-dried to obtain a layered double metal hydroxide.
[0060] The XRD spectrum ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com