Near-infrared absorption phosphorescent material based on Ir(ttp)-aza-BODIPY, preparation method and application thereof
A technology of heterofluoroboron dipyrrole and tetramethyl porphyrin is applied in the field of near-infrared absorbing phosphorescent materials, which can solve the problems of short luminescence life and achieve the effect of long phosphorescence decay life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1. compound A:
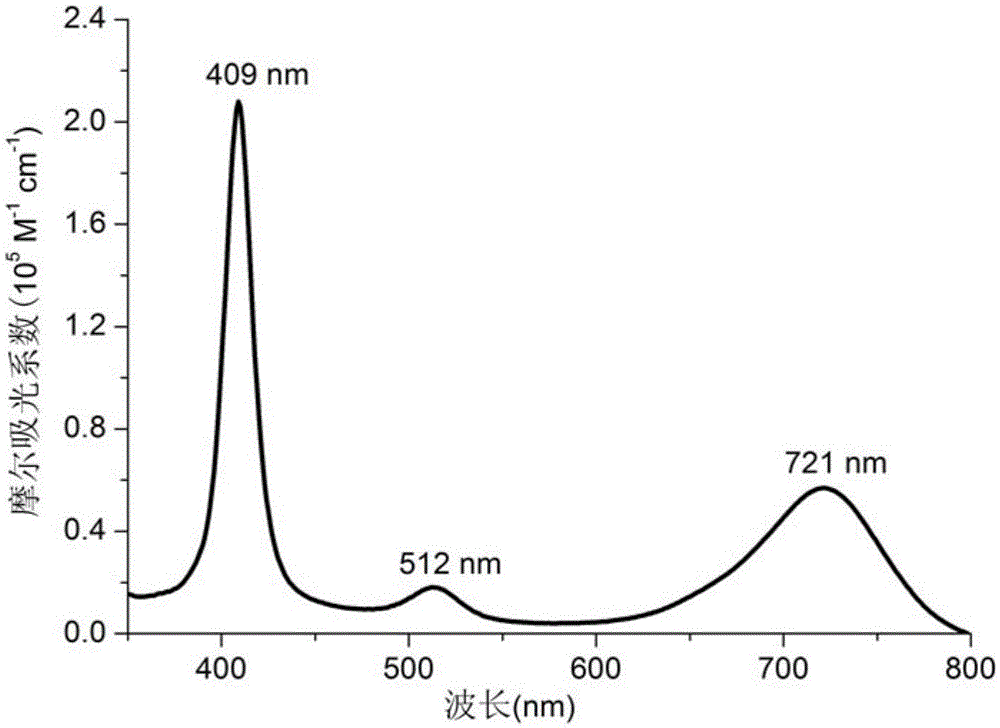
[0026] Carbonyl chloride·tetracresyliridium porphyrin Ir(ttp)(CO)Cl (15.8mg, 0.017mmol), brominated azafluoroboron dipyrrole aza-BODIPY-a (13.7mg, 0.021mmol) were added to the reactor ) (12.3mg, 0.019mmol), K 2 CO 3 (47.0mg, 0.34mmol) and 1.0mL of benzene, the reaction mixture was refrigerated and degassed three times, and reacted at 150°C for 72 hours under the protection of nitrogen. After the reaction is finished, the solvent is spin-dried under reduced pressure, and the 2 Cl 2 / Hexane (1:1) as a developing agent for silica gel column chromatography (100-140 mesh, Qingdao Ocean Chemical Factory) to obtain 11.4mg of compound B. Yield: 46%.R f =0.64(CH 2 Cl 2 / hexane=1:1). 1 HNMR (400MHz,C 6 D. 6 ):δ1.27(d,2H,J=8.3Hz),2.41(s,12H),5.48(s,1H),5.83(d,2H,J=8.7Hz),6.28(s,1H),6.91 (d, 3H, J=7.9Hz), 7.02-7.08(m, 5H), 7.23(d, 4H, J=7.4Hz), 7.35(d, 4H, J=7.3Hz), 7.41-7.47(m, 4H), 7.72(d, 2H, J=7.5Hz), 7.93(d, 4H, J=7.4Hz),...
Embodiment 2
[0027] Embodiment 2. Synthesis of compound B:
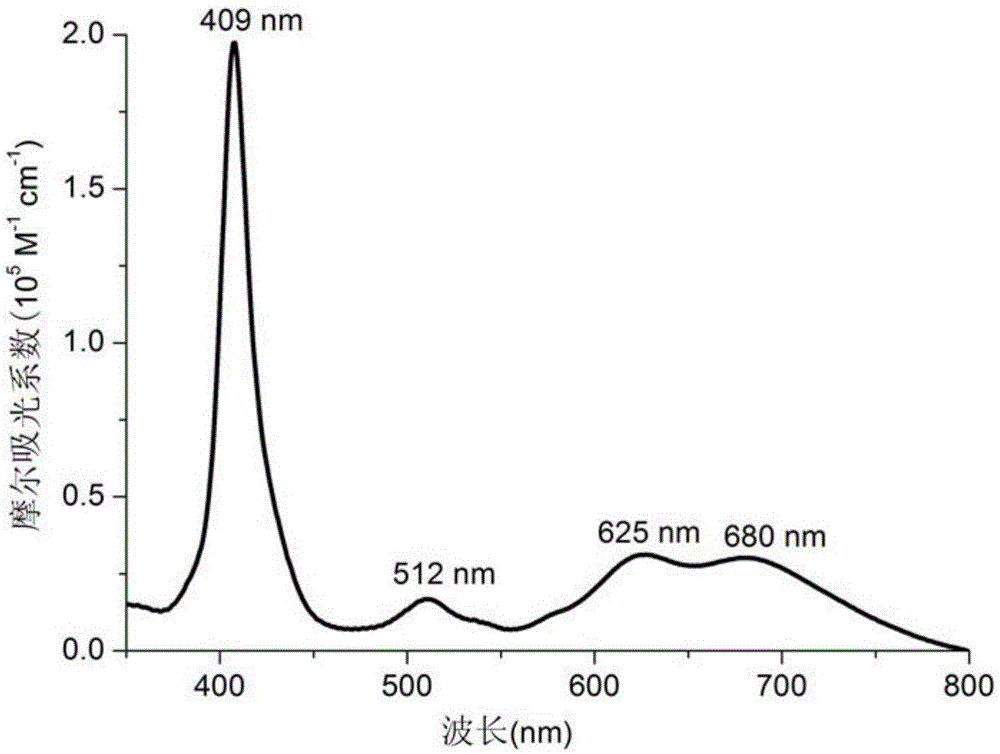
[0028] Add carbonyl chloride·tetracresyl iridium porphyrin Ir(ttp)(CO)Cl (18.0mg, 0.019mmol), bromoazafluoroboron dipyrrole aza-BODIPY-b (13.7mg, 0.021mmol) into the reactor ), K 2 CO 3 (53.9mg, 0.39mmol) and 1.0mL of benzene, the reaction mixture was refrigerated and degassed three times, and reacted at 150°C for 48 hours under the protection of nitrogen. After the reaction is finished, the solvent is spin-dried under reduced pressure, and the 2 Cl 2 / Hexane (1:1) as a developing agent for silica gel column chromatography (100-140 mesh, Qingdao Ocean Chemical Factory) separation, to obtain 17.1 mg of compound A. Yield: 61%.R f =0.68(CH 2 Cl 2 / hexane=1:1). 1 HNMR (400MHz,C 6 D. 6 ):δ1.12(d,2H,J=8.4Hz),2.42(s,12H),5.40(d,2H,J=4.3Hz),5.55(s,1H),6.35(s,3H),6.86 -7.05(m,4H),7.26-7.31(m,6H),7.38-7.43(m,6H),7.69(d,2H,J=5.8Hz),7.91(d,2H,J=7.7Hz), 8.01(d, 4H, J=7.6Hz), 8.22(d, 4H, J=7.6Hz), 8.97(s, 8H).; HRMS(FABMS): Calcdfor...
Embodiment 3
[0029] Example 3. Application
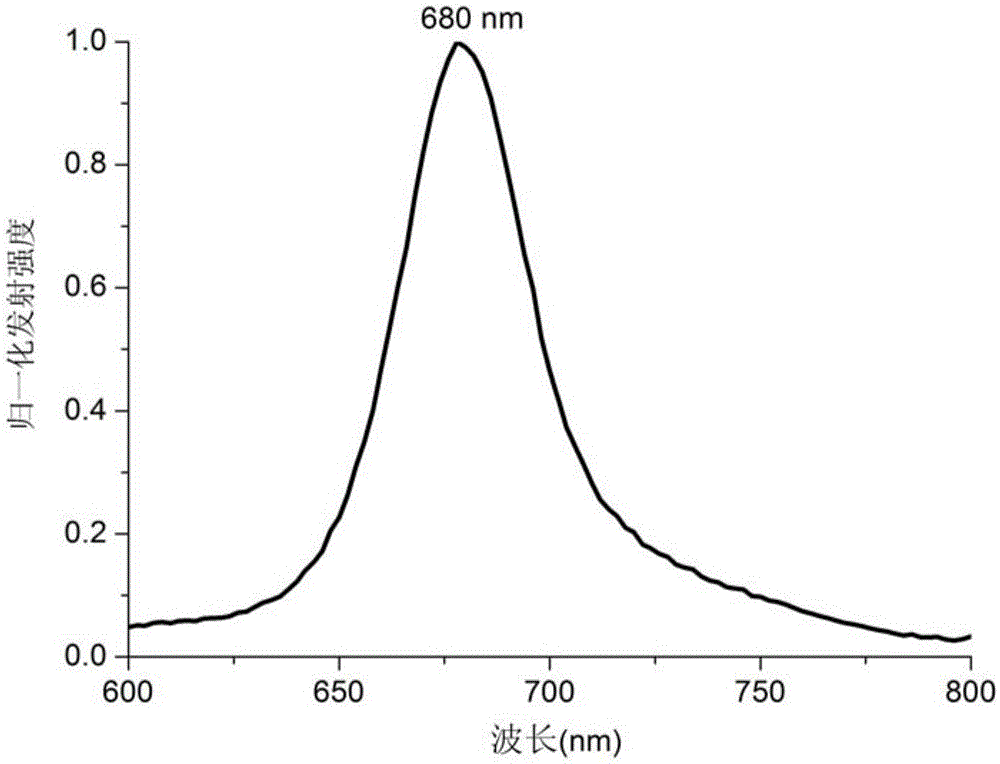
[0030] We tested the stability of the target compound by using ultraviolet spectroscopy, using methylene blue as a reference, using the efficiency value of a 671nm laser singlet oxygen, and a monochromatic laser with a wavelength of 690nm as a light source. The experimental results show that the synthesized target compound has good stability and high singlet oxygen generation efficiency, and can be successfully applied to photodynamic therapy. We carried out the MTTassay test of photosensitizer A in HeLa cells in the presence of light and in the absence of light to detect its cytotoxicity. The experimental results show that under the condition of no light source, with the increase of the photosensitizer concentration, the activity of the cells basically does not change and maintains at about 100%. The results show that the cells do not die in the dark in the presence of the photosensitizer. In contrast, under the irradiation of 690nm laser ligh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com