Novel triterpenoid compound, preparation method and medical application thereof
A compound and composition technology, applied in the field of new compounds, can solve the problems of killing tumors, low immune function, toxic and side effects, etc., and achieves the effects of mild process conditions, killing tumor cells, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
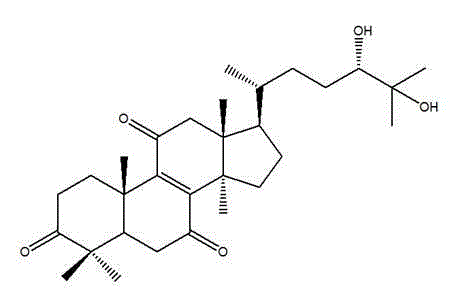
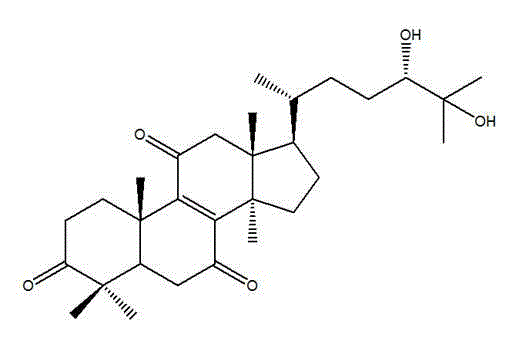
[0036] Example 1 Formula (Ⅰ) Compound preparation
[0037] Material source Ganoderma ( Ganodermalucidum (Leys.exFr.) Karst) was purchased from Fujian Xianzhilou Biotechnology Co., Ltd., China. The specimen of the Ganoderma lucidum is deposited in the School of Pharmacy of Fujian Medical University.
[0038] Extraction and separation The dried and crushed Ganoderma lucidum was refluxed and extracted with absolute ethanol for 3 times, 2 hours each time, the extract was filtered with No. 2 filter paper, and the ethanol was removed with a rotary evaporator to obtain an alcohol extract. After adding appropriate amount of water to the alcohol extraction extract, it was extracted with petroleum ether and ethyl acetate successively, and the obtained ethyl acetate extract was extracted with saturated sodium bicarbonate aqueous solution. The ethyl acetate phase was taken out under reduced pressure and evaporated to dryness to obtain a crude product. Column chromatography was performed o...
Embodiment 2
[0039] Example 2 Formula (Ⅰ) Chemical structure determination of compounds
[0040] Structure determination Use Shimadzu-3100 spectrophotometer to measure the ultraviolet spectrum, in CDCl 3 In the solution, the NMR spectrum was recorded with a BRUKER nuclear magnetic resonance spectrometer, and the mass spectrum was measured with an Agilent 6210 time-of-flight mass spectrometer.
[0041] Formula (Ⅰ) The physical and chemical properties of the compound The compound of formula (I) of the present invention is white crystal, UV (EtOH) λmax254nm; Liebermann-Burchard reaction is positive; 1 H-NMR (CDCl 3 , 500MHz): δ 1.74 (1H, m, H-1), δ 2.95 (1H, m, H-1'), δ 2.47 (1H, m, H-2), δ 2.58 (1H, m, H-2'), δ 2.24 (1H, m, H-5), δ 2.36 (1H, m, H-6), δ 2.52 (1H, m, H-6'), δ 2. 63(1H,m,H-12),δ2.71(1H,m,H-12'),δ1.62(1H,m,H-15),δ2.13(1H,m,H-15' ), δ 1.20 (1H, m, H-16), δ 2.10 (1H, m, H-16'), δ 1.65 (1H, m, H-17), δ 0.81 (3H, s, H-18), δ 1.26 (3H, s, H-19), δ 1.40 (1H, m, H-20), δ 0.89 (3H, d, ...
Embodiment 3
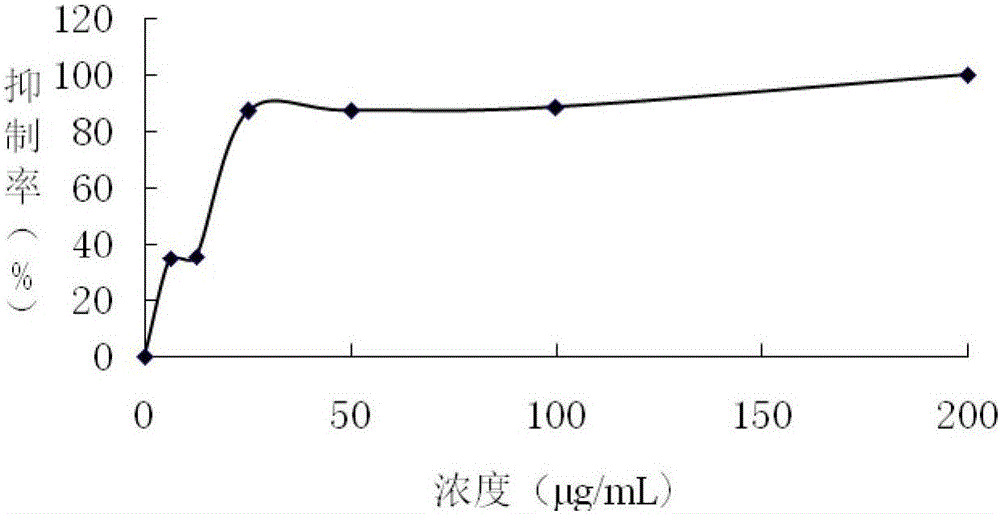
[0044] Example 3 Formula (Ⅰ) Biological experiment and analysis of compound anticancer effect
[0045] 1. Materials and methods
[0046] Cell lines and reagents Human esophageal cancer cell line OE-19 and human myeloid leukemia cell line HL60. These cells were cultured in RPMI1640 medium containing 10% calf serum, and placed in CO at 37°C and 5% saturated humidity. 2 Cultivate in an incubator. The above-mentioned triterpenoid formula (Ⅰ) Compound-chemical name 24(S)-24,25-dihydroxylanost-8-en-3,7,11-trione, from Obtained from the preparation of Example 1.
[0047] Cell proliferation analysis Take the OE-19 and HL60 cells in logarithmic growth phase and inoculate them in a 96-well culture plate at a certain density according to the cell line, 190 μl per well. Add medicine immediately after inoculating the cells. The experimental group was added with different concentrations of drugs 10μl / well, the cell control group was supplemented with serum-free culture medium containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com