Codon optimized phosphocholine cytidylyltransferase gene and expression thereof
A technology of codon optimization and phosphorylcholine, applied in the field of microorganisms, can solve the problems of low conversion rate, cumbersome process and high production cost, and achieve the effect of high crude enzyme liquid enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
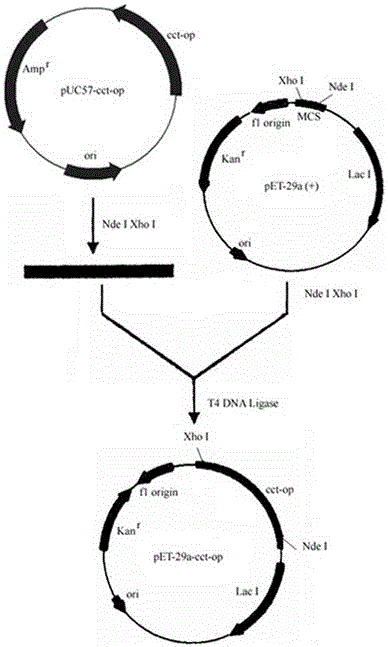
[0025] Example 1 Construction of recombinant Escherichia coli.
[0026] 1.1 Codon optimization Codon optimization and whole gene synthesis of phosphorylcholine cytidine transferase gene.
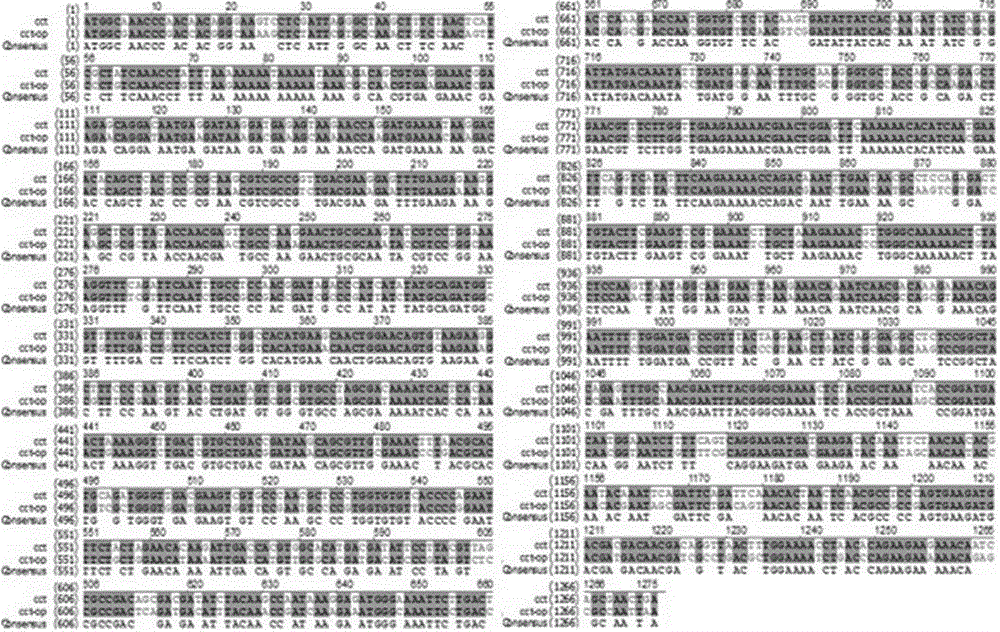
[0027] The present invention is based on the phosphorylcholine cytidine transferase derived from Saccharomyces cerevisiae cct The gene sequence adopts the method of whole gene synthesis to optimize the phosphorylcholine cytidine transferase gene according to the preferred codon of Escherichia coli, and entrusts Nanjing Tuoda Biotechnology Co., Ltd. to synthesize it. The pUC57 plasmid is used as a subcloning vector in the gene synthesis service for Phosphocholine cytidine transferase gene The open reading frame of the phosphorylcholine cytidine transferase gene synthesized by codon-optimized preference in Escherichia coli is 1275 bases. The homology comparison was carried out by VectorNTI software, and it was found that the homology between the synthetic sequence and the original sequence wa...
Embodiment 2
[0034] Example 2 Inducible expression of codon-optimized phosphorylcholine cytidine transferase.
[0035] 2.1 Induced expression of recombinant bacteria BL21(DE3) / pET-29a-cct-op and Rosetta(DE3) / pET-29a-cct-op.
[0036] Strain activation: Streak the recombinant bacteria L21(DE3) / pET-29a-cct-op and Rosetta(DE3) / pET-29a-cct-op on the solid plate containing kanamycin LB on the clean bench A single colony was isolated and cultured overnight at 37°C in a constant temperature incubator.
[0037]Seed culture: Under sterile conditions, pick a single colony on the plate, insert it into a test tube of 3 mL of LB liquid medium added with kanamycin, and culture it on a shaker at 37°C for 12 hours at a constant temperature of 200rpm.
[0038] Induced expression: Transfer the seed solution to a 50ml Erlenmeyer flask (250ml) containing LB medium containing kanamycin at a 2% (v / v) inoculation amount. Proliferate at 220 rpm at 37°C for 2.5-3h to OD 600 To 0.6-0.8, add IPTG at a final concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com