Method for generating oxygen, water electrolysis device and anode
A water electrolysis and anode technology, applied in electrodes, electrode coatings, electrolysis processes, etc., can solve the problem of no disclosure of the method of generating oxygen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
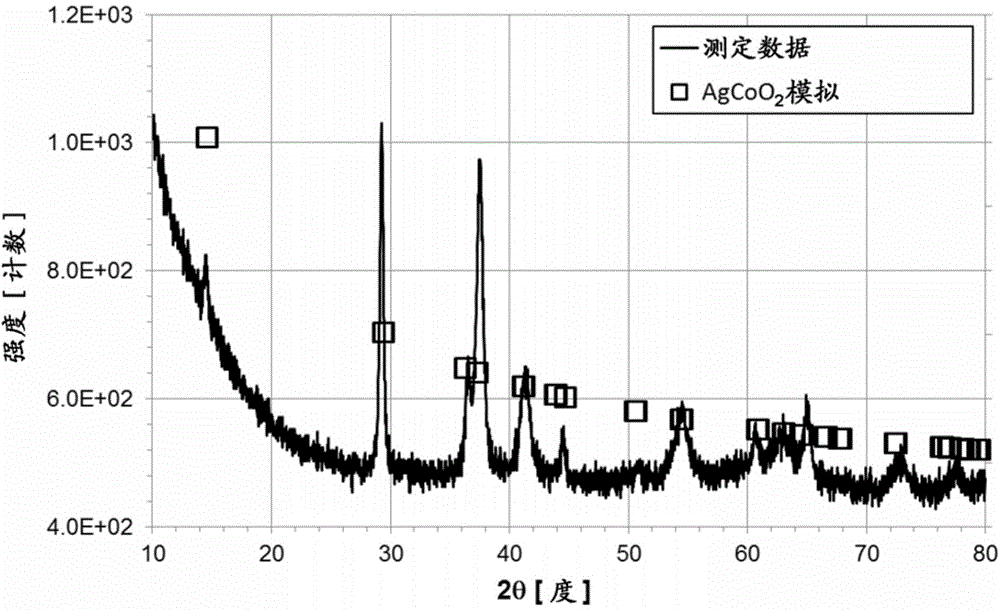
[0064] In Embodiment 1, as the delafossite-type silver compound, AgCoO 2 Represented delafossite-type silver-cobalt compounds.
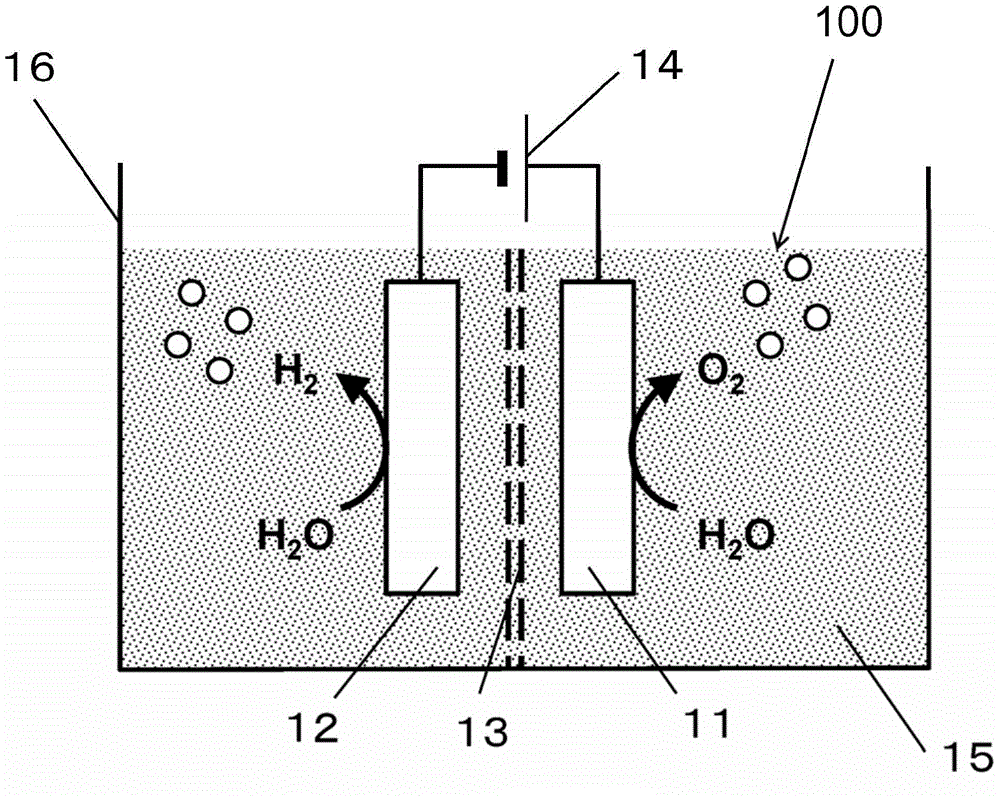
[0065] figure 1 A schematic diagram of the water electrolysis device 100 according to Embodiment 1 is shown. The water electrolysis device 100 according to Embodiment 1 includes a container 16 , an anode 11 , a cathode 12 , and a power source 14 .
[0066] (container 16)
[0067] An aqueous electrolyte solution 15 is stored inside the container 16 . An example of the aqueous electrolyte solution 15 is an alkaline aqueous solution such as potassium hydroxide or sodium hydroxide. By electrolyzing water using an alkaline aqueous solution, the efficiency of oxygen generation can be improved and the electric power required for electrolysis can be reduced.
[0068] Other examples of the electrolyte contained in the aqueous electrolyte solution 15 include sulfuric acid, nitric acid, or perchloric acid. More specifically, examples of cations of the ele...
Embodiment approach 2
[0094] In Embodiment 2, as the delafossite-type silver compound, AgRhO 2 Represented delafossite-type silver-rhodium compounds.
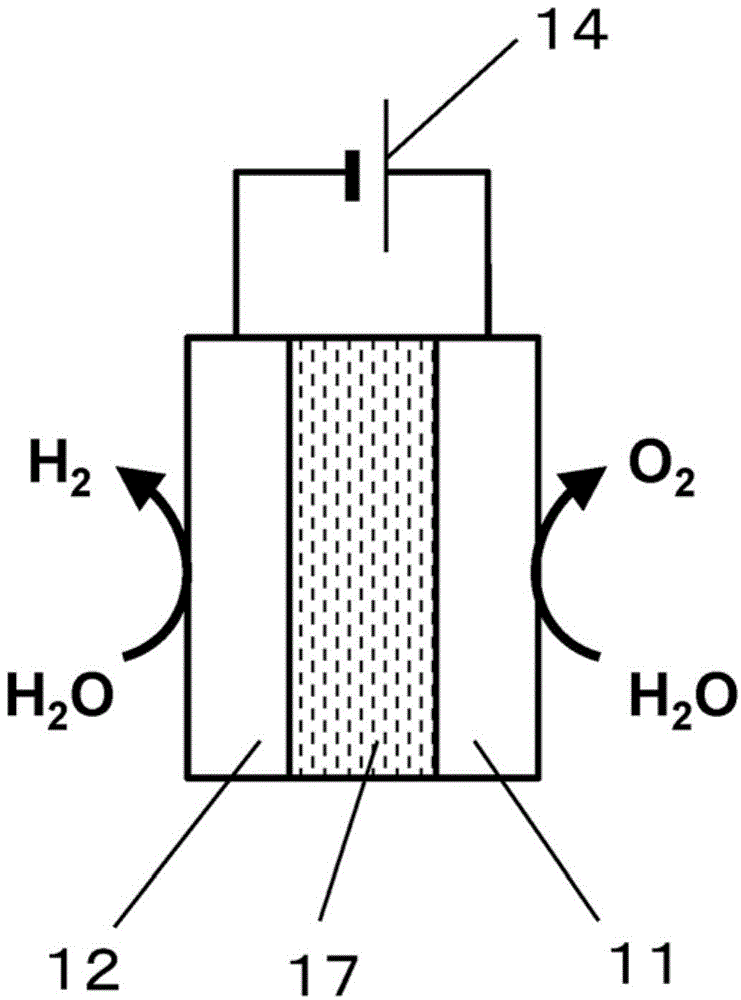
[0095] Figure 5 It is a schematic diagram of the water electrolysis device 200 of Embodiment 2. Figure 5 The shown water electrolysis device 200 includes a container 21 , an anode 22 , a cathode 23 , and a power source 24 .
[0096]
[0097] An aqueous electrolyte solution 25 is accommodated in the container 21 . Examples of the aqueous electrolyte solution 25 include alkaline aqueous solutions such as potassium hydroxide and sodium hydroxide. By electrolyzing water using an alkaline aqueous solution, it is possible to increase the efficiency of oxygen generation and reduce the power required for electrolysis.
[0098] Other examples of the electrolyte contained in the aqueous electrolyte solution 25 include sulfuric acid, nitric acid, or perchloric acid. More specifically, examples of cations of the electrolyte contained in the aqueous ele...
Embodiment 1
[0129] (production of anode 11)
[0130] The anode 11 of Example 1 was produced by supporting a delafossite-type silver-cobalt compound on a conductive carbon substrate.
[0131] First, the delafossite-type silver-cobalt compound was prepared by hydrothermal synthesis.
[0132] Specifically, the chemical formula Co(OH) will be 2 The indicated cobalt hydroxide (available from Wako Pure Pharmaceutical Co., Ltd.) was heated at a temperature of 120° C. in an oxygen atmosphere for 24 hours to obtain cobalt oxyhydroxide represented by the chemical formula CoOOH. Cobalt oxyhydroxide (0.25 g) represented by the chemical formula CoOOH and the chemical formula Ag 2 Silver oxide represented by O (available from Wako Pure Pharmaceutical Co., Ltd., 0.63 g) was mixed in a sodium hydroxide solution (2 mol / L, 40 mL) to obtain a mixture.
[0133] The mixture was heated in a Teflon (registered trademark) vessel at a temperature of 210° C. for 60 hours to obtain a product.
[0134] The obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com