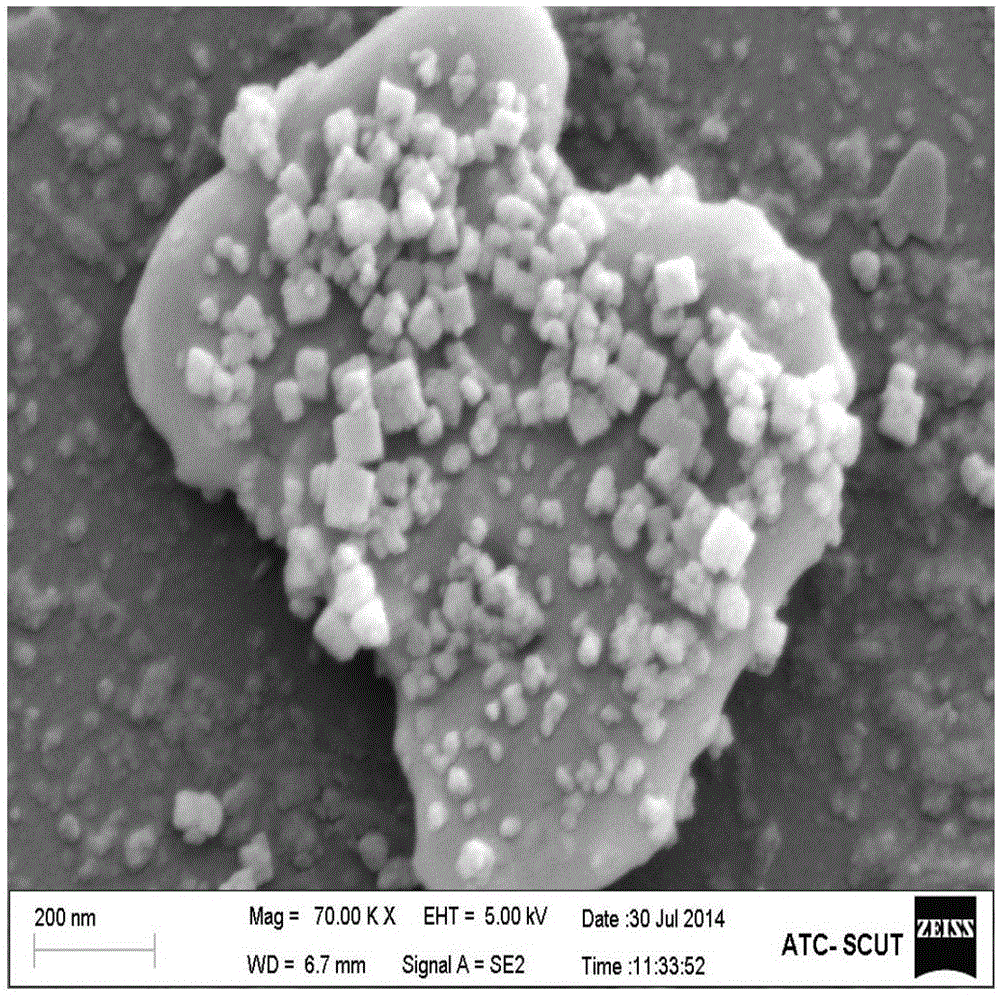
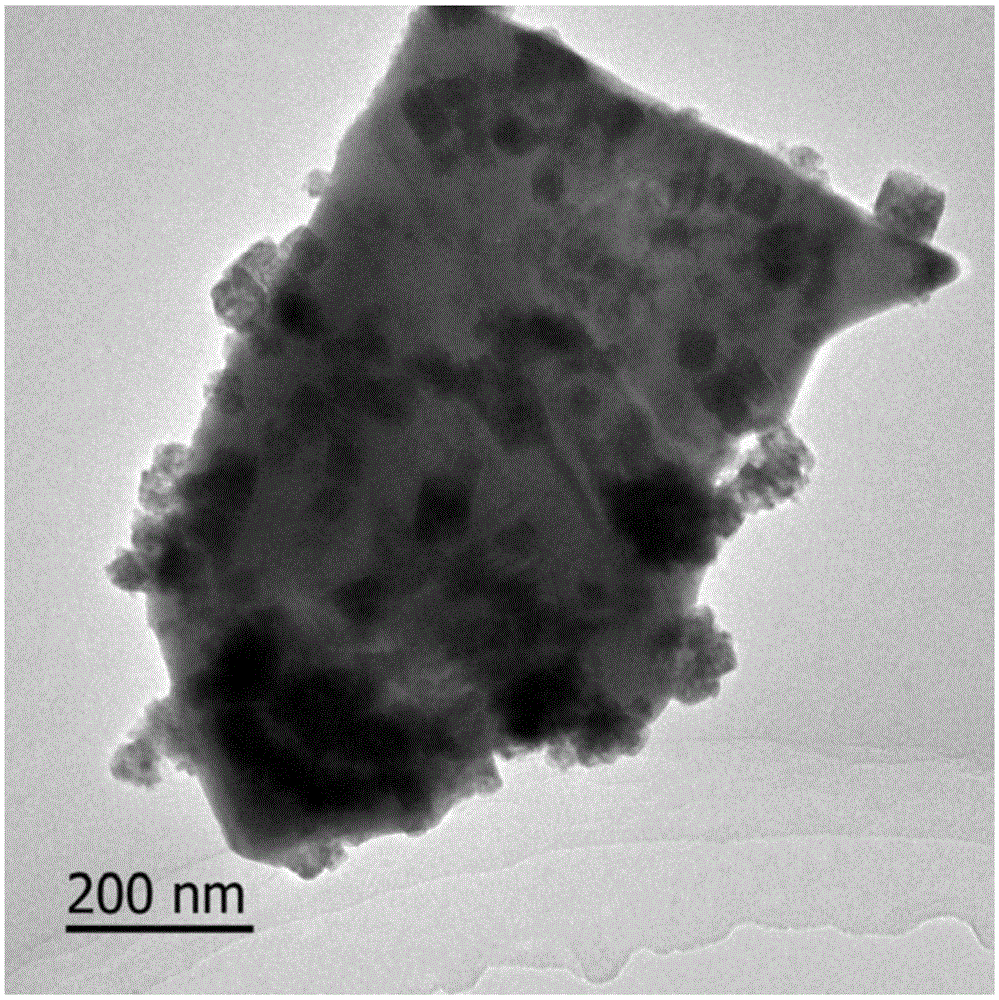
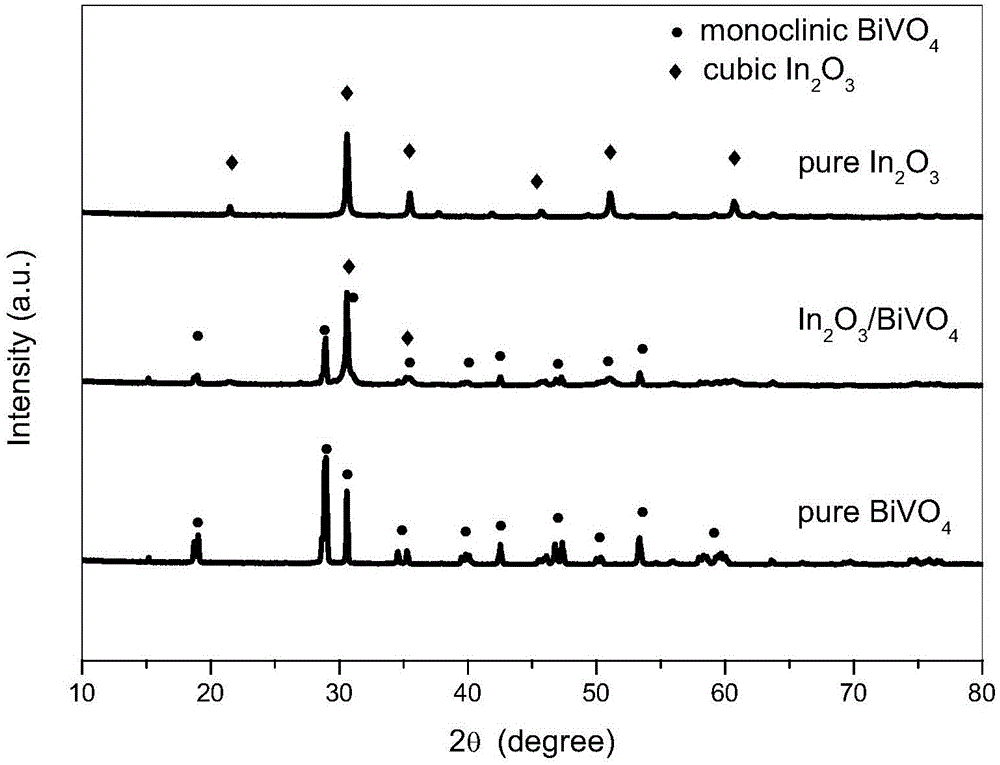
Indium oxide-bismuth vanadate compound photocatalyst as well as preparation method and application of photocatalyst
A technology of bismuth vanadate and indium oxide, which is applied in the field of inorganic nano-photocatalyst materials, can solve the problems of limiting the photocatalytic performance of bismuth vanadate, the difficulty of separating photogenerated carriers, and the easy recombination of electrons and holes, and achieve the best visible light response ability , large specific surface area, and the effect of improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under normal temperature and pressure, weigh 2.42g of Bi(NO 3 ) 3 ·5H 2 O and 1.95g of In(NO 3 ) 3 ·5H 2 O powder in beaker A, add 20mL of nitric acid with a concentration of 4M, stir well to obtain a clear solution; and weigh 0.58g of NH 4 VO 3 In beaker B, add 20mL of NaOH with a concentration of 4M, stir well to obtain a clear solution; then, under rapid stirring, add the solution in beaker A to the solution in beaker B dropwise to obtain an orange-yellow precipitate, add The concentration is 1M NaOH solution, the pH value is adjusted to 7, and after fully stirring for 30 min, the orange solution is transferred to a 100 mL hydrothermal reaction kettle, and the hydrothermal reaction is carried out at 180° C. for 24 h. After cooling to room temperature, the product was washed several times with deionized water, then placed in an oven at 60°C for 24 hours, treated in a mortar after cooling, placed in a muffle furnace at 500°C, and calcined for 2 hours. After cool...
Embodiment 2
[0031] Under normal temperature and pressure, weigh 4.82g of Bi(NO 3 ) 3 ·5H 2 O and 3.90g of In(NO 3 ) 3 ·5H 2 O powder in beaker A, add 20mL of nitric acid with a concentration of 4M, stir well to obtain a clear solution; and weigh 1.16g of NH 4 VO 3 In beaker B, add 20mL of NaOH with a concentration of 4M, stir well to obtain a clear solution; then, under rapid stirring, add the solution in beaker A to the solution in beaker B dropwise to obtain an orange-yellow precipitate, add The concentration is 1M NaOH solution, the pH value is adjusted to 7, and after fully stirring for 30 min, the orange solution is transferred to a 100 mL hydrothermal reaction kettle, and the hydrothermal reaction is carried out at 180° C. for 24 h. After cooling to room temperature, the product was washed several times with deionized water, then placed in an oven at 60°C for 24 hours, treated in a mortar after cooling, placed in a muffle furnace at 500°C, and calcined for 2 hours. After cool...
Embodiment 3
[0033] Under normal temperature and pressure, weigh 4.82g of Bi(NO 3 ) 3 ·5H 2 O and 3.91g of In(NO 3 ) 3 ·5H 2 O powder in beaker A, add 20mL of nitric acid with a concentration of 4M, stir well to obtain a clear solution; and weigh 1.16g of NH 4 VO 3 In beaker B, add 20mL of NaOH with a concentration of 4M, stir well to obtain a clear solution; then, under rapid stirring, add the solution in beaker A to the solution in beaker B dropwise to obtain an orange-yellow precipitate, add The concentration is 1M NaOH solution, the pH value is adjusted to 7, and after fully stirring for 30 min, the orange-yellow precipitate is transferred to a 100 mL hydrothermal reaction kettle, and the hydrothermal reaction is carried out at 180 ° C for 24 h. After cooling to room temperature, the product was washed several times with deionized water, then placed in an oven at 60°C for 24 hours, treated in a mortar after cooling, placed in a muffle furnace at 500°C, and calcined for 2 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com