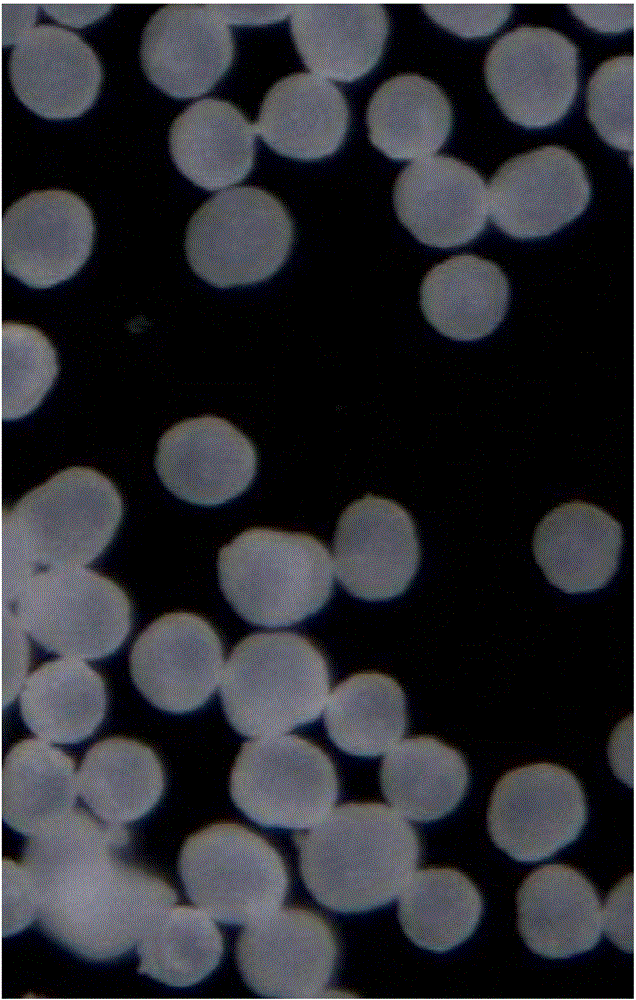
Preparation method of clopidogrel hydrogen sulfate I crystal form spherical crystal
A technology of clopidogrel bisulfate and clopidogrel free base, which is applied in organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulas, etc., and can solve the problems of inconvenient solvent recycling, complicated process, long time, high Solvent recovery and recycling, simple solvent removal process, and easy solvent residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Acquisition of seeds
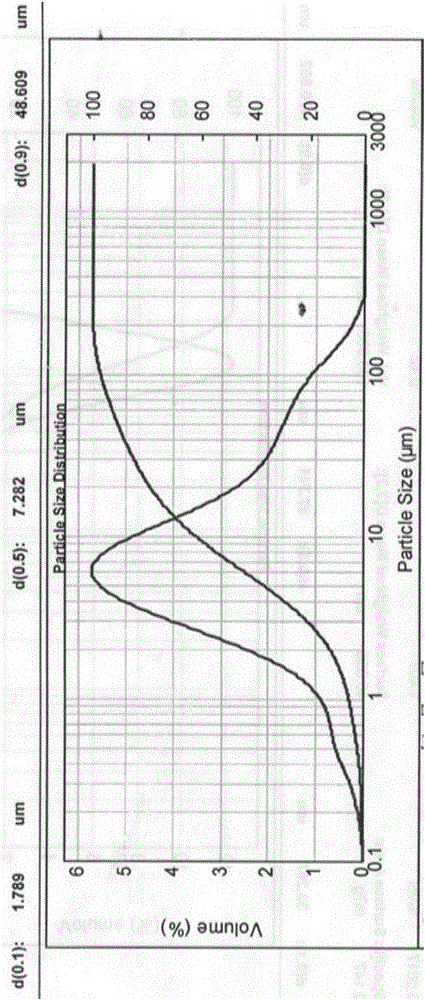
[0058] Clopidogrel hydrogen sulfate I crystal form (purity greater than 99.0%) is pulverized with an MX50-II type airflow mill, and the air pressure is 5 to 6 atm to obtain clopidogrel hydrogen sulfate seed crystal D50=7.3 μm, and its particle size distribution is as follows: figure 1 Shown (MalvernMastersizer2000 particle size analyzer).
[0059] Seed crystals with a specific particle size distribution can be obtained through conventional pulverization process control means in the field, such as air flow pressure, pulverization time, sieving, and the like.
Embodiment 2
[0061] Take 760g of clopidogrel bisulfate (purity greater than 99.0%) and add it into 10L of dichloromethane, add 5L of water and solid sodium bicarbonate until the pH of the aqueous phase>7. Stand still for liquid separation, take the organic phase and wash with water (1L×2), and remove water with anhydrous magnesium sulfate until the solution is clear.
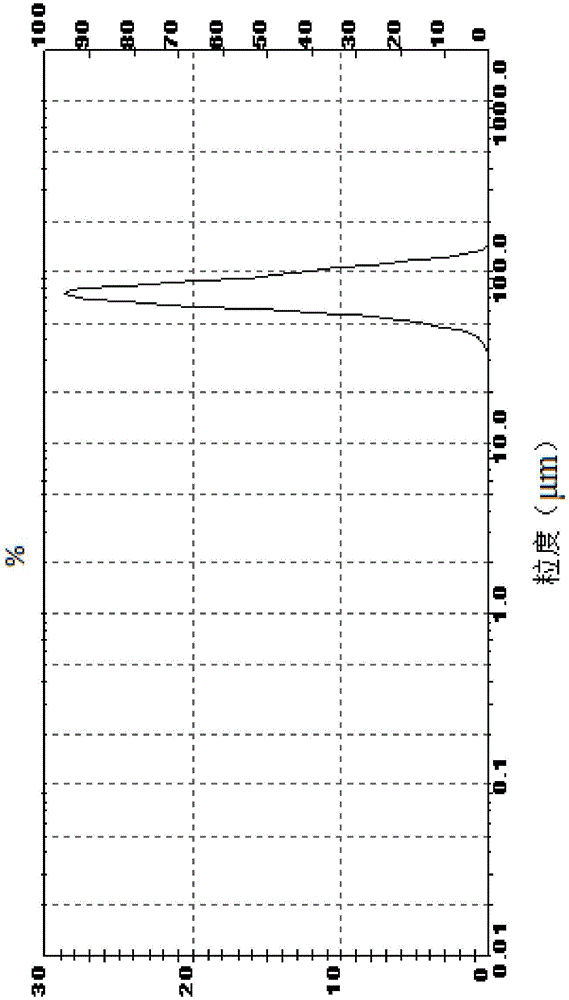
[0062] The organic phase was filtered and vacuum rotary evaporated until the quality did not change, the residue was dissolved in 10.5 L of 2-butanol, and the solution was kept at 20°C. 100 mL of concentrated sulfuric acid (98%, 181 g) was dispersed in 2.5 L of 2-butanol and added to the system within 60 minutes. The temperature was raised to 25° C., and 5.8 g (about 1% wt relative to clopidogrel free base) of the crystal form I obtained in Example 1 (D50=7.3 μm) was added under rapid stirring. Keep warm at 25°C for 2.5h, then cool down to 15°C and keep warm for 4h. Suction filtration, the filter cake was washed with ethyl...
Embodiment 3
[0065] Take 1000 g of clopidogrel camphorsulfonate (purity greater than 99.0%) and add it to 10 L of dichloromethane, add 5 L of water and solid sodium bicarbonate until the pH of the aqueous phase>7. Stand still for liquid separation, take the organic phase and wash with water (1L×2), and remove water with anhydrous magnesium sulfate until the solution is clear.
[0066] The organic phase was filtered and vacuum rotary evaporated until the mass did not change, the residue was dissolved in 9L of 2-butanol, and the solution was kept at 20°C. 100 mL of concentrated sulfuric acid (98%, 181 g) was dispersed in 3 L of 2-butanol and added to the system within 60 minutes. The temperature was raised to 25° C., and 17.4 g (about 3% wt relative to clopidogrel free base) of Form I seed crystal (D50=10 μm) obtained in Example 1 was added under rapid stirring. Keep warm at 25°C for 3h, then cool down to 15°C and keep warm for 4.5h. Suction filtration, the filter cake was washed with ethy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com