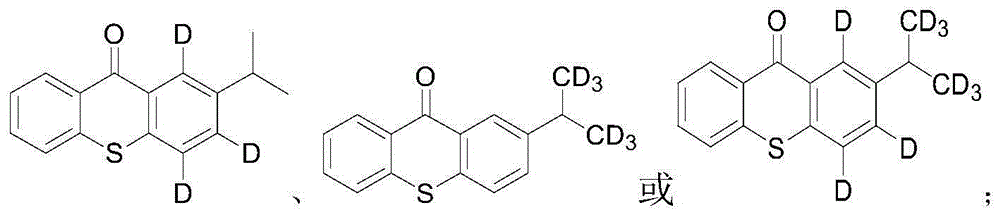
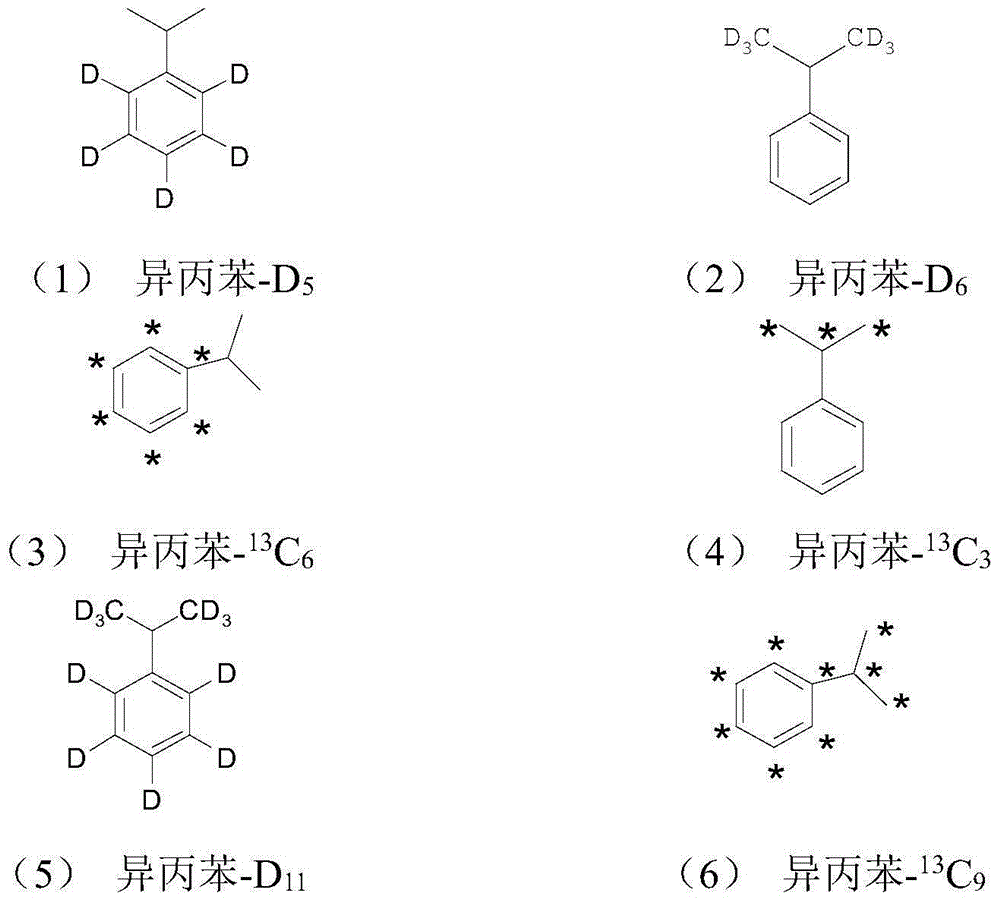
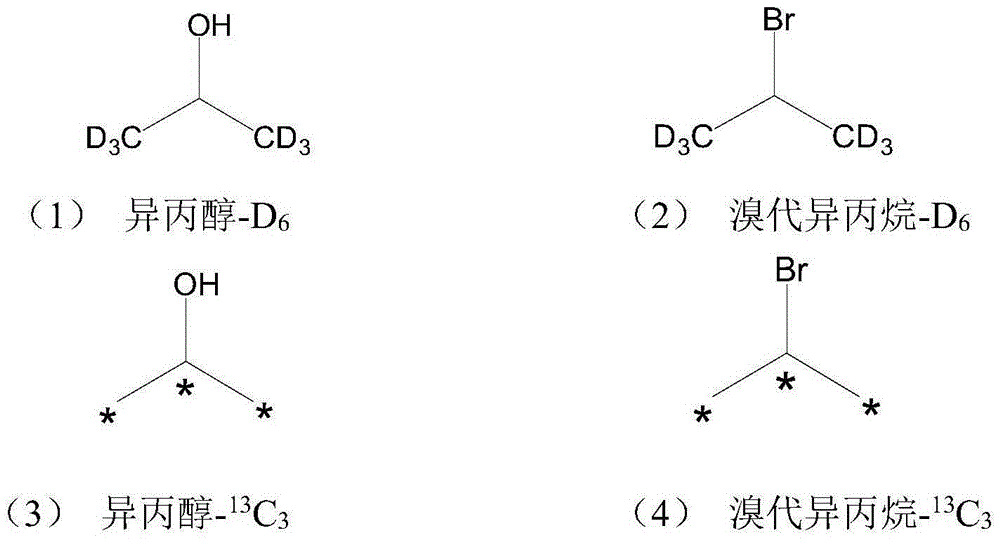
Stable isotopic labeled 2-isopropylthioxanthone and synthetic method thereof
A technology of isopropylthioxanthone and stable isotopes, applied in the field of stable isotope labeling of 2-isopropylthioxanthone and its synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] A. Stable isotope labeled bromobenzene-D 5 Synthesis
[0073] Add N-bromosuccinimide (NBS), anhydrous ferric chloride (its molar ratio is 1:0.1) successively in the flask, with molar ratio NBS: benzene-D 6 = 2:1 add benzene-D 6 , and acetonitrile with a reaction volume of 20%, stirred, heated to 100°C, and reacted for 6h. After the reaction, ether extraction, washing, anhydrous Na 2 SO 4 Drying, rectification under reduced pressure (20KPa, 160°C) to get bromobenzene-D 5 , the yield is 62.6% (by dropping into benzene-D 6 ), the product has a chemical purity of 98.3% through gas phase quantitative detection, and an abundance of 99.6atom%D through GC-MS / MS.
[0074] B. Stable isotope labeled cumene-D 5 Synthesis
[0075] Add metal magnesium to the flask, operate without water and oxygen, N 2 Protected, under stirring, add stable isotope-labeled bromobenzene-D with a syringe 5 , metal magnesium and bromobenzene-D 5 The molar ratio is 1:1, and the reaction volume ...
Embodiment 2
[0080] A. Stable isotope labeled bromobenzene-D5 Synthesis
[0081] Add dibromohydantoin (DBDMH), anhydrous ferric tribromide (its molar ratio is 1:0.1) successively in the flask, with molar ratio NBS: benzene-D 6 = 3:1 add benzene-D 6 , and acetonitrile with a reaction volume of 20%, stirred, heated to 90°C, and reacted for 7h. After the reaction, ether extraction, washing, anhydrous Na 2 SO 4 Drying, rectification under reduced pressure (20KPa, 160°C) to get bromobenzene-D 5 , the yield is 63.9 (in order to drop into benzene-D 6 ), the product has a chemical purity of 97.9% through gas phase quantitative detection, and an abundance of 99.6atom%D through GC-MS / MS.
[0082] B. Stable isotope labeled cumene-D 5 Synthesis
[0083] Add metal magnesium to the flask, operate without water and oxygen, N 2 Protected, under stirring, add stable isotope-labeled bromobenzene-D with a syringe 5 , metal magnesium and bromobenzene-D 5 The molar ratio is 2:1, and the reaction volu...
Embodiment 3
[0088] A. Stable isotope labeled bromobenzene-D 5 Synthesis
[0089] Ammonium tribromide (TBAB) and anhydrous ferric chloride (wherein the molar ratio of ammonium tribromide to anhydrous ferric chloride is 1:0.1) are added successively in the flask, with the molar ratio NBS:benzene-D 6 =4:1 add benzene-D 6 , and acetonitrile with a reaction volume of 20%, stirred, heated to 80° C., and reacted for 8 hours. After the reaction, ether extraction, washing, anhydrous Na 2 SO 4 Drying, rectification under reduced pressure (20KPa, 160°C) to get bromobenzene-D 5 , the yield is 63.4% (in order to drop into benzene-D 6 ), the product has a chemical purity of 98.0% through gas phase quantitative detection, and an abundance of 99.6atom%D through GC-MS / MS.
[0090] B. Stable isotope labeled cumene-D 5 Synthesis
[0091] Add metal magnesium to the flask, operate without water and oxygen, N 2 Protected, under stirring, add stable isotope-labeled bromobenzene-D with a syringe 5 , me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com